Non-cytolytic enterovirus
Non-cytolytic enterovirus is an aberrant form of enterovirus able to cause chronic infection. Normally enteroviruses only produce acute infections, but mutations to the virus acquired in the host can convert enterovirus B serotypes such as coxsackievirus B and echovirus into non-cytolytic viruses able to create persistent intracellular viral RNA infections.
This viral RNA infection self-replicates very slowly, and does not readily destroy the cells it inhabits, but nevertheless synthesizes viral proteins and may cause pathological effects. Persistent non-cytolytic enterovirus is found in a number of chronic illnesses, from myalgic encephalomyelitis to type 1 diabetes, and some researchers postulate this non-cytolytic form of the virus plays a causal role in these diseases.
Non-cytolytic enterovirus is also known as non-cytopathic enterovirus, and defective enterovirus.
Introduction
Non-cytolytic enterovirus is an aberrant form of viral infection enterovirus B serotypes (such as coxsackievirus B and echovirus) can transmute into within the host, a transformation that allows a virus normally only capable of acute infections to create persistent ones. Molecular evidence for the existence of this form of infection dates back to at least 1990, but an understanding of the molecular mechanism behind the conversion of lytic to non-cytolytic enterovirus was only obtained in 2005, when Professor Nora Chapman figured out the mechanism.
The transformation of normal lytic (wild-type) enterovirus into the non-cytolytic form results from mutations (specifically, deletions in the genome) the virus acquires in the host during the first weeks of acute infection. This altered form of enterovirus may then cause persistent intracellular infections which replicate very slowly without killing the cells the virus resides in. Non-cytolytic enterovirus infections it is thought may lead to cellular dysfunction, affect immune signaling, induce autoimmunity, or elicit a pro-inflammatory immune response. Non-cytolytic enterovirus is difficult for the immune system to eradicate, so it can persist for very long periods, and is associated with a range of chronic diseases.
Persistent enteroviral infections have been found in:
- The brain, muscle and stomach tissues of myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) patients; see enterovirus infection studies.
- The heart muscle in murine and human coxsackievirus B (CVB) chronic myocarditis.[1][2]
- The heart muscle in dilated cardiomyopathy (a sequelae of myocarditis).[3][4][5][6][7][8][9][10][11]
- The cerebrospinal fluid and spinal cord tissue of amyotrophic lateral sclerosis (a motor neuron disease).[12][13][14]
- The brainstem in Parkinson's disease.[15]
- The salivary gland epithelial cells and lymphocyte infiltrates in primary Sjögren's syndrome.[16]
- The cerebrospinal fluid in post-polio syndrome.[17]
- The murine pancreas[18] and human pancreas,[19][20] which has implications for type 1 diabetes.
- The intestines in ileocecal Crohn's disease.[21]
- The heart valve tissues in valvular heart disease.[22][23]
- The calf muscle in peripheral arterial disease.[24]
- The stomach tissues in functional dyspepsia and chronic gastritis.[25]
The persistent enterovirus infections found in the above diseases are in several cases (those shown in italics) explicitly demonstrated to be non-cytolytic. Several researchers including Prof Nora Chapman, Prof Steven Tracy and Dr John Chia theorize that persistent non-cytolytic enterovirus infection may be a cause of ME/CFS. Persistent enterovirus is being investigated as a possible cause for the other above-listed diseases as well.
Non-cytolytic enterovirus is also known as non-cytopathic enterovirus. Cytolytic refers to destruction of a cell by rupture of its membrane, and cytopathic signifies damage to a cell, typically by rupture. Thus the terms non-cytolytic and non-cytopathic indicate this mutated enterovirus does not usually destroy the cells in which it resides. Non-cytolytic enterovirus may also be referred to as defective enterovirus and terminally deleted enterovirus, terms which refer to the mutations (deletions) in its genome which render this virus defective and aberrant.
The nature of non-cytolytic infection
When enterovirus is initially contracted by a host, it creates an acute lytic infection. Lytic infection involves the virus entering into host cells and replicating rapidly, producing thousands of new viral particles (virions) in each infected cell, then rupturing and killing the cell through lysis, allowing the new virions to escape and infect more cells. The immune response will clear such acute infections from the host within weeks. Until relatively recently, enterovirus was thought only capable of acute lytic infection; it was not generally believed to cause chronic infection.[26][27]
However, it is now clear that during the first weeks of acute infection, enterovirus B serotypes can sometimes transmute into the non-cytolytic form — a form which can then go on to create persistent infections in the host.[28] This form is so named because cell destruction by lysis is rarely seen in the cells it infects. The conversion from lytic to non-cytolytic enterovirus results from certain mutations (deletions at the end of the viral genome) which may manifest during the acute phase.[29][1] Once these mutations occur, it changes the capabilities of the virus, producing a modified pathogen that is now able to evade the immune system, and form persistent infections.
Non-cytolytic infections are not a different species of enterovirus; they arise from regular enterovirus B serotypes in common circulation, but which as a result of these mutations get transmuted into a distinct quasispecies. Viral quasispecies are viruses which within the host have acquired small mutations in their genome, but which are still closely related to their parent virus. In the case of non-cytolytic enterovirus, these acquired mutations lead to a dramatically altered virus lifecycle.[30]
The lifecycle of a regular lytic enterovirus centers on the virion, but once transformed to a non-cytolytic infection, the virus then exists in a very different configuration: as strands of naked viral RNA that reside within host cells creating a persistent intracellular RNA infection. This intracellular viral RNA is self-replicating and self-sustaining, and is thought can survive independently of any help from the lytic infection. Furthermore, whereas the lytic virus destroys by lysis the cells it infects, non-cytolytic enterovirus does not typically kill the host cells it inhabits, allowing the non-cytolytic infection to reside in these cells on a long-term basis.
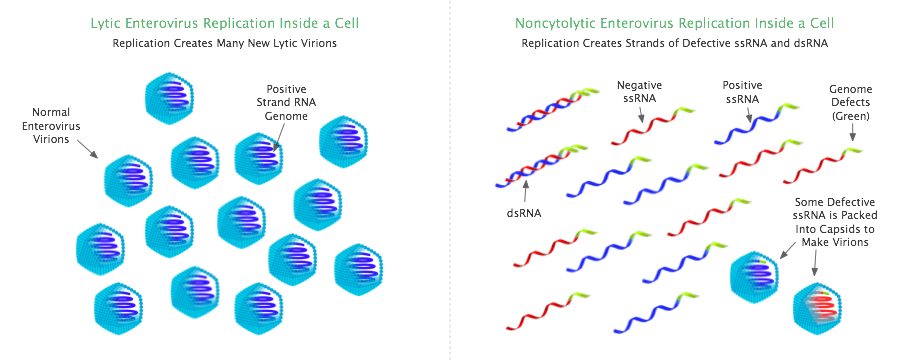
The self-sustaining RNA infection of non-cytolytic enterovirus consists of positive and negative single-stranded viral RNA (ssRNA), as well as double-stranded viral RNA (dsRNA).[31] The latter is formed when the positive ssRNA and negative ssRNA in the cell join to create dsRNA.
Non-cytolytic infections very rarely produce lytic virions (infectious virions with a complete genome), neither in chronic myocarditis nor dilated cardiomyopathy[3][9] nor ME/CFS.[32][33] Non-cytolytic infection can nevertheless propagate by packing its defective genomes into capsids (viral shells) to create virions; this is one way the defective virus can spread.[34]
Note that the non-cytolytic state of enterovirus is distinct from viral latency: in the latent state, viruses are typically dormant for long periods and do not engage in viral replication or viral propagation; whereas a non-cytolytic infection actively replicates (albeit very slowly) and propagates.
RNA viruses such as enterovirus are generally not capable of latency (usually only DNA viruses are able to go into latency). So latency is not a mechanism that enterovirus could employ to create a persistent presence in the host. But it is now clear enterovirus can form a chronic low-level intracellular infection as a non-cytolytic defective virus and can persist in immunocompetent hosts for very long periods.[35]
Non-cytolytic enterovirus is resistant to immune elimination
Non-cytolytic enterovirus can cause chronic long-lasting infections, as its mutated RNA is not readily cleared by the immune system.[36] The reason why this infection can evade the immune response is not clear.[37]
One theory is that the double-stranded viral RNA (dsRNA) component of this infection confers resistance to immune eradication. The immune enzyme RNase L, released inside the cell as part of the type 1 interferon response to viral infection, is able to destroy (cleave) ssRNA, but dsRNA is resistant to destruction by this enzyme.[38][39]
Dr John Chia likens dsRNA to a seed, which is hardy and allows the non-cytolytic infection to survive through periods of immune attack. Afterwards when the pressure of the immune response abates, he thinks it is probable that the dsRNA can dissociate back into ssRNA and recommence replication. In this way, the dsRNA may constantly reseed the non-cytolytic infection. Dr Chia points out that self-replicating strands of RNA or DNA are well studied, and are known as replicons.[40][41]
Another theory suggested by Lévêque et al[42] is that the deletions found in the non-cytolytic enterovirus genome might facilitate immune evasion: alphaviruses are known to utilize such deletions within their genome to evade the type 1 interferon-induced immune response, and non-cytolytic enterovirus has deletions in exactly the same area of the genome as alphavirus. The deletions in the alphavirus genome prevent interferon-induced Ifit1 immune proteins from binding to and disabling alphavirus RNA.[43] If the genomic mutations of non-cytolytic enterovirus also confer the same immune resistance, that may help explain how this non-cytolytic virus evades immune clearance.
Non-cytolytic enterovirus may cause pathogenic effects leading to disease
In the cells it infects, non-cytolytic enterovirus synthesizes the full range of viral proteins, and these viral proteins may interfere with cellular function or cause pathology.[44]
For example, enterovirus 2A protein, a protein produced by non-cytolytic enterovirus, has been shown in a murine model to generate dilated cardiomyopathy just on its own.[45] The 2A protein cleaves dystrophin, decreases the contractility of heart cardiomyocyte cells, as well as increasing cell membrane permeability; and 2A can promote virus spread to adjacent cells.[46] Enteroviral protein has been found in human heart tissues in dilated cardiomyopathy,[47] as well as in the skeletal muscles, stomach and brain tissues of ME/CFS. In vitro studies indicate that non-cytolytic enteroviral RNA synthesizes similar amounts of viral protein as wild-type lytic enterovirus (though non-cytolytic viral RNA with the largest deletions generates less protein).[48]
Furthermore, viral dsRNA is a potent inducer of type 1 interferons,[49] so even the low levels of dsRNA found in non-cytolytic infections may be sufficient to chronically induce interferon, which might then lead to adverse effects (although it remains to be seen if these low levels are capable of stimulating type I interferon secretion,[50] and one study suggests they may not, at least in murine heart muscle cells).[51]
Non-cytolytic enterovirus infection in the heart muscle in chronic CVB myocarditis and dilated cardiomyopathy has also been shown in some studies to induce autoantibodies which target mitochondria and thus substantially inhibit energy metabolism.[52][53][54][55][56] Article
How does lytic enterovirus transmute into the non-cytolytic form?
The mechanism by which lytic (wild-type) enterovirus can get transformed into a non-cytolytic virus within the host was identified by Prof Nora Chapman in a landmark 2005 study.[57]
Chapman says that the conversion of acute lytic enterovirus into a non-cytolytic infection can only occur in specific cell types, namely in non-dividing quiescent cells (such as muscle cells), but cannot occur in dividing cells (like liver cells).
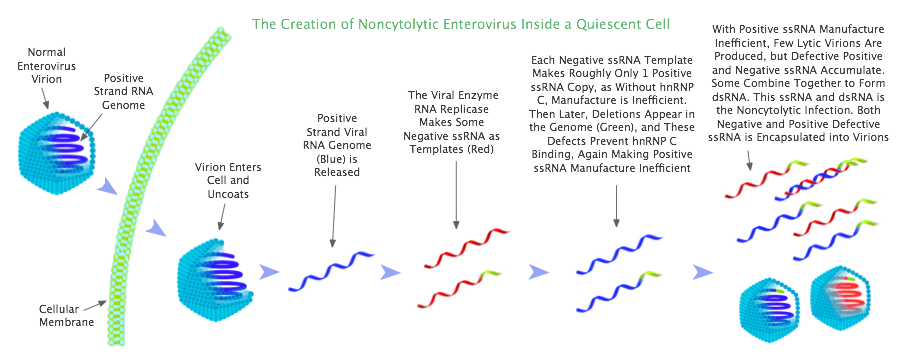
A certain amount of non-cytolytic enterovirus RNA is always generated during lytic replication, through random mutations from replication errors; but in dividing cells, because production of lytic virus is efficient, lytic virus populations dominate over non-cytolytic populations, and the cell thus is co-opted for lytic replication. By contrast, in quiescent cells, due to specific cellular conditions, lytic virus manufacture is very inefficient, and this provides an opportunity for non-cytolytic populations in quiescent cells to grow and prevail. Once non-cytolytic virus establishes itself as the dominant species in the cell, it co-opts the cell for non-cytolytic replication. This transmutation of lytic into non-cytolytic enterovirus occurs during acute infection, in a timeframe in the order of weeks after the virus is first caught.
This is the essence of how non-cytolytic enterovirus infection is born, but we now explain in detail how this transmutation from lytic to non-cytolytic virus occurs.
In the mechanics of lytic enterovirus infection, viral negative single-stranded viral RNA (ssRNA) is employed as a template to create numerous positive ssRNA copies (like a photographic negative producing lots of prints). The positive ssRNA copies are then packed into capsids (viral shells) to make new enterovirus virions. Enteroviruses are positive single-stranded RNA viruses, meaning their genome comprises positive ssRNA; so in order to create thousands of new enterovirus virions, thousands of copies of the positive ssRNA are required.
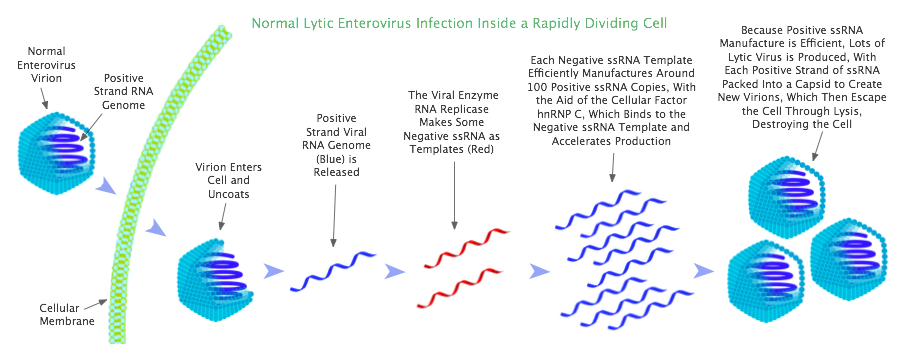
When enterovirus enters a rapidly dividing cell, it efficiently produces thousands of copies of positive ssRNA with the help of an important factor present in the cell known as hnRNP C (heterogeneous nuclear ribonucleoprotein C). This factor works by binding to the negative ssRNA, where it has the effect of accelerating production of positive ssRNA.[58]
With the aid of hnRNP C, each strand of enterovirus negative ssRNA is able to efficiently produce around 100 strands of positive ssRNA. So in rapidly dividing cells, a 100-fold excess of positive strands over negative strands is observed.
However, in a quiescent cell, the factor hnRNP C is not available to the virus, as this factor is confined to the cell nucleus when cells are not dividing. Only when a cell starts dividing (mitosis) does hnRNP C move into the cytoplasm (the region between the nucleus and the cell membrane), where enterovirus replicates. Without the assistance of hnRNP C, positive ssRNA production is severely impacted, and now each negative ssRNA template is only able to manufacture in the order of 1 strand of positive ssRNA. In consequence, roughly equal numbers of positive and negative ssRNA strands are observed in quiescent cells.
Because of the great shortfall of positive ssRNA in quiescent cells, very few lytic virions are created. As a result, cellular lysis — the destruction of the cell as the virions are released — does not occur. Instead the cell survives, and is populated with roughly equal amounts of enteroviral positive and negative ssRNA.
With these conditions found in quiescent cells, a non-cytolytic enterovirus infection can emerge. The emergence pivots on natural defects appearing in the viral genome. In viral replication, during the synthesis of viral RNA, reproduction errors naturally arise; these errors will often be in the form of deletions in the 5′ (pronounced "five primed") region at the terminal end of the genome (5′ is an untranslated region at the end of the genome). These deletions just result from premature termination of the process of genome transcription.[59]
Now it just so happens that these deletions in the 5′ region are located in precisely the part of the genome that hnRNP C binds to on the negative ssRNA template.[60] Thus once the deletions occur in the virus, this genomic defect permanently prevents efficient production of positive ssRNA.
So now, not only do we have a cell which being quiescent lacks cytoplasmic hnRNP C, but this situation is compounded by the deletions in the viral genome, which prevent hnRNP C binding to the negative strand template. Under these conditions, lytic virus creation greatly inhibited, and the replication process starts duplicating the genomes containing deletions, ie, duplicating the non-cytolytic virus. These circumstances favor the evolution and domination of non-cytolytic enterovirus,[61] and lead to the creation of a non-cytolytic infection.
This transmutation of lytic into non-cytolytic infection by means of genome deletions cannot occur in a dividing cell, because although viral replication in dividing cells produces RNA with the same genomic deletions, these deleted genomes are outnumbered by the high amounts of lytic virions efficiently generated, thanks to hnRNP C. And furthermore, during lytic virus production in a dividing cell, the process rapidly kills the cell by lysis, so the deleted genome RNA infection has nowhere to live. Thus conversion to the non-cytolytic form is thought possible only in quiescent cells.
Deletions in the 5′ terminal end of the viral genome in persistent low-level coxsackievirus B infections were first detected by Nora Chapman and colleagues in the 2005 study,[57] and have since been demonstrated in subsequent studies.[62][1][63] The deletions range from 7 to 49 nucleotides in length[30] (by comparison the full coxsackievirus B genome comprises around 7,400 nucleotides).[42]
Lévêque et al[42] speculate that these genome deletions might also facilitate immune evasion, thereby making non-cytolytic enterovirus largely invulnerable to immune clearance.
So this is the mechanism and conditions by which non-cytolytic enterovirus infection is born, and once this intracellular infection is created, it is observed that the immune system cannot easily clear it.
Factors that promote the creation of a non-cytolytic infection
The question arises: why do these persistent non-cytolytic infections not always occur after acute coxsackievirus B infection? What circumstances lead to acute lytic enterovirus transforming into a chronic non-cytolytic infection within the host?
Prof Nora Chapman suggests that it probably requires a high level of acute infection to seed quiescent cells with enterovirus, such that a non-cytolytic infection has a chance to emerge.[64]
Interestingly, Dr Chia found that when corticosteroids are inadvertently given to patients during the acute stage of an enterovirus infection, this is a recipe for triggering enterovirus-associated ME/CFS. Corticosteroids suppress the antiviral immune response, and may thus cause a higher level of acute infection.
The spread of non-cytolytic enterovirus infection in the host
Non-cytolytic enterovirus, though defective due to deletions in its genome, appears capable of propagating itself within the host. This is demonstrated when cultured non-cytolytic enterovirus is injected intraperitoneally into mice: the infection travels to the heart, and then produces a persistent non-cytolytic infection in the heart tissues.[65]
Non-cytolytic enterovirus is able to propagate because although the virus is defective, its genome is still packaged into capsids to make virions which can transport the non-cytolytic infection to other cells. Evidence for this virion mode of propagation comes from the fact that when cultured non-cytolytic coxsackievirus B3 is introduced to a cell line, infection of those cells can be prevented by CVB3 neutralizing antibody.
Non-cytolytic enterovirus virions may be transmitted to other cells by means of extracellular vesicles, the formation of cell protrusions, and intercellular bridges.[66]
Interestingly, when non-cytolytic enteroviral RNA is packed into virions, as well as virions containing the normal positive ssRNA genome, negative ssRNA is also encapsidated in almost as many virions. Thus in non-cytolytic infection, virions containing the negative ssRNA anti-genome are found, which is very unusual, and this is a signature of non-cytolytic infection.[67][57]
Detection of non-cytolytic infections
Non-cytolytic infection replicates at too low a level for detection by cytopathic effect, but non-cytolytic enterovirus can be detected in tissues samples by sensitive reverse transcription-PCR (RT-PCR) and by immunohistochemistry.[68]
In their 1999 study Tam and Messner[39] used RT-PCR to detect the non-cytolytic enteroviral RNA in the tissues, with different primers to distinguish positive from negative viral ssRNA. In order to detect the dsRNA, they first used the RNase I enzyme to destroy the viral ssRNA in the tissue, after which a positive result from RT-PCR testing indicates the presence of dsRNA.
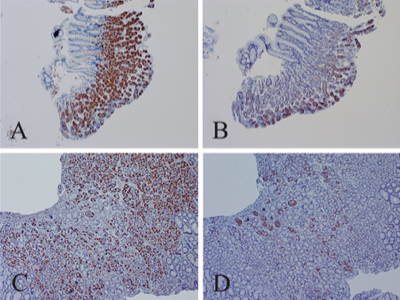
For the immunohistochemistry detection of non-cytolytic enterovirus, Dr John Chia uses the 5-D8/1 monoclonal antibody stain for highlighting enterovirus VP1 protein, and the J2 monoclonal antibody stain to reveal the dsRNA component of non-cytolytic infection.[25] Chia employs such tissue staining to detect non-cytolytic enterovirus infections in ME/CFS patients' stomach tissue biopsies. In a study, Chia found enterovirus VP1 protein in 82% of ME/CFS patients' stomach biopsies, compared to 20% of the controls.[33]
Indirect evidence of chronic enterovirus infection is suggested by the persistently elevated coxsackievirus B or echovirus antibody titers often observed in ME/CFS patients with these infections. However these raised antibody titers are usually only detected when sensitive techniques such as plaque reduction neutralization testing or micro-neutralization testing are employed. Complement fixation testing (CFT) is not sensitive enough to detect antibody titers in chronic enterovirus infections (CFT is only useful in the first few months of initial infection).[69][70]
Dr Chia measured the antibody titers of 200 consecutive ME/CFS patients and 150 controls, using the ARUP Lab CVB and echovirus antibody micro-neutralization tests, and found the patients' titers were often substantially higher than the mean titers of the controls.[71][72] Highly elevated CVB titers may also be seen in a small subset of dilated cardiomyopathy patients.[73]
PCR blood testing is not sufficiently sensitive for the chronic enteroviral infections found in ME/CFS, because enteroviral RNA is only present at low levels in the blood, Dr Chia discovered. Chia determined that with special techniques and repeated testing, enterovirus RNA can be detected in around 30% of whole blood samples taken from chronically infected enterovirus-associated ME/CFS patients.[74] This finding emerged from Chia's lab work in which over a 5 year period he tested more than 2,500 blood samples from over 510 ME/CFS patients by PCR. Chia observed that only 35% of patients tested positive twice or more for enteroviral RNA in their peripheral blood lymphocytes (PBL), using a highly sensitive semi-nested RT-PCR test kit from Chemicon (with an assay sensitivity of 80 to 800 copies of RNA per ml of blood). In the same RT-PCR test 4% of the controls tested positive. Interestingly, in these RT-PCR blood tests, Chia found enterovirus RNA in 70% of the severe bedridden patients, but only in 12% of the less ill.[75][76]
Furthermore, when 20 of the sickest patients' PBL cells were retested every 3 to 6 months for 2 years, Chia found that patients who had tested positive might be negative on the next test, and then might be positive again on a subsequent test; he observed it was rare for a patient with a positive PCR result to consistently test positive. Chia concluded it was clear that the enteroviral RNA present in the blood is at very low levels.[77] Dr Chia says any PCR blood tests which have insufficient sensitivity (of above 1000 copies of RNA per ml of blood) will almost always give negative results in ME/CFS patients.[78]
Note that when Dr Chia was aiming to detect non-cytolytic enterovirus infection in the brain tissue of a deceased ME/CFS patient, on RT-PCR testing he initially got a negative result. Dr Chia then remembered a phenomenon whereby viral RNA may bind to chromosomal DNA, which prevents the RT-PCR from detecting the viral RNA. So Dr Chia then used the DNase enzyme to digest the chromosomal DNA in the brain tissue, and after doing this, he was able to find enterovirus RNA in various brain areas.[79]
Treatment of non-cytolytic infections
Interferon alpha therapy has been found temporarily effective for enterovirus-associated ME/CFS in some studies,[80][81][82] with treatment often resulting in large improvements in symptoms. In one experiment by Dr John Chia, ribavirin plus interferon alpha therapy resulted in 7 out of 10 enterovirus ME/CFS patients obtaining substantial improvements in symptoms, elimination of enteroviral RNA from their peripheral blood mononuclear cells (PBMC) and a fourfold reduction in their coxsackievirus B antibody titers. However, relapse typically occurred 4 to 5 months after therapy was completed, along with antibody titers increasing to pretreatment values and enteroviral RNA returning to the PBMC.
In another experiment by Dr Chia, interferon alpha plus interferon delta were used in combination to treat severe bedbound ME/CFS patients with chronic enterovirus infections: 6 out of 11 treated patients were able to return to full- or part-time work as a result, but again relapse occurred some months later; the relapse was typically triggered by a bout of heavy exertion. Thus interferon therapy does not appear to be a long-term solution for ME/CFS; however, the large improvements many enterovirus ME/CFS patients experience after interferon therapy does provide some evidence that enterovirus is playing an ongoing causal role in ME/CFS. Further details: interferon therapy for ME/CFS.
Clearly better treatments are required, and Dr Chia says there is an urgent priority to develop inhibitors for viral RNA replicase, the main mechanism for RNA replication which facilitates the persistence of non-cytolytic enteroviral RNA in infected cells.[82]
Summary characteristics of non-cytolytic enterovirus infection
Non-cytolytic enterovirus infection:
- Is a self-sustaining intracellular infection of naked viral RNA possessing genomic deletions which are responsible for transmuting the original lytic virus into the non-cytolytic form.
- Is born in non-dividing (quiescent) cells such as muscle cells, where cellular conditions promote the evolutionary selection of non-cytolytic virus defective genomes in preference to intact lytic virus genomes.
- Is made of positive and negative single-stranded viral RNA as well as double-stranded viral RNA. This RNA replicates very slowly and at a low level. The dsRNA may contribute to pathogenicity of non-cytolytic infection.
- Is likely capable of replicating independently of the lytic enterovirus infection; tissues infected with non-cytolytic enterovirus are often largely devoid of lytic virus.
- Is able to synthesize all the normal viral proteins in the host cell, which may alter cellular functioning and cause pathogenicity.
- Is resistant to the immune response, and can thus reside inside host cells for very long periods. Its double-stranded RNA form is postulated to confer resistance to immune clearance; other theories suggest the genomic deletions furnish the non-cytolytic virus with the ability to evade the immune response.
- Is not cytolytic or cytopathic: there is no significant destruction of cells (a marker of lytic infection) either in vivo or when non-cytolytic infections are cultured in vitro (hence the names non-cytolytic and non-cytopathic). The presence of enteroviral RNA in the tissues in the absence of cell destruction is a signature of non-cytolytic enterovirus infection.
- Is able to encapsidate its defective genome into capsids to create viral particles (virions), enabling non-cytolytic virus to spread. Just as many negative strand anti-genomes are encapsidated as positive strand genomes, and the presence of negative strand virions is a signature of non-cytolytic enterovirus infection.
- Is observed to have a reduced positive to negative single-stranded RNA ratio: the ratio in acute lytic infection is around 100:1 (around 100 times more positive strand than negative strand), but in persistent non-cytolytic infection the ratio is closer to 1:1. Such reduced ratios are a signature of non-cytolytic enterovirus infection.
A brief history of non-cytolytic enterovirus
In the lifecycle of an acute lytic enterovirus infection, this virus will enter a host cell, replicate into thousands of new virions, destroying the cell by lysis in the process. However enterovirus, like most RNA viruses, is not capable of assuming a latent state within cells, and enterovirus infections are generally considered to be acute and rapidly cleared by the host immune response.[26][27] Indeed, John Chia points out that even today, most physicians are taught that enterovirus does not form chronic infections.[83]
Thus it was puzzling why coxsackievirus B appeared capable of forming persistent infections in the heart muscle tissues in chronic CVB myocarditis and dilated cardiomyopathy, and in the skeletal muscle tissues in ME/CFS. Enteroviral RNA would be detected in these tissues, indicating an ongoing enterovirus infection, but infectious lytic virus can almost never be isolated from the adult heart in chronic myocarditis,[84] nor from the skeletal muscles in ME/CFS. (Lytic virus is detected by its cytopathic effect: when infected tissue samples are added to a cell culture in vitro, if lytic enterovirus is present it will lyse the cells in culture and this cytopathic effect can be observed; but a cytopathic effect is very rarely seen with chronic myocarditis, dilated cardiomyopathy and ME/CFS tissue samples).
This was the conundrum for decades: how can enteroviral RNA be persistently present in these tissues, but without any lytic virus present? This paradox was eventually resolved by the discovery and understanding of the non-cytolytic form of enterovirus, which can create chronic intracellular infections of self-replicating enteroviral RNA, but with very little lytic virus generated.
Some studies in non-cytolytic enterovirus research
Within a few years of their discovery in the late 1940s, the Coxsackie B viruses were shown to be involved in myocarditis,[85] but direct evidence that enterovirus was able to maintain a persistent infective presence in the heart muscle tissues would have to wait until molecular testing techniques became available in the late 1980s which could detect the virus in the tissues by its RNA. Once these molecular techniques were employed, they not only demonstrated that enteroviral RNA is chronically present in the tissues, but they led to an intriguing further discovery: that in persistent enterovirus tissue infections, the ratio of positive to negative strand enteroviral RNA was greatly reduced compared to acute enterovirus infection. This prompted the speculation that persistent enterovirus infections might involve a virus with a mutated defective genome.
Fifteen years later, these genomic defects were first detected by Nora Chapman, Steven Tracy, Kyung-Soo Kim, William Tapprich and colleagues in a landmark 2005 paper.[30] The defects were in the form of deletions in the 5′ region of the genome. In this same paper, Chapman identified the precise molecular mechanism by which these acquired genome defects were able to transform lytic enterovirus (which normally only causes acute infections) into a virus capable of very long-term viral persistence in the tissues. Later studies confirmed the presence of these deletions in the 5′ region in other chronic enterovirus human heart infections, thus providing supporting evidence for Chapman's proposed mechanism.
- Archard et al 1987: [3] using molecular hybridization testing detected Coxsackie B virus RNA in 56% of the heart tissue biopsies from myocarditis or dilated cardiomyopathy, whereas biopsies from controls with non-viral heart diseases were all negative. The authors also found viral RNA in the skeletal muscles of patients with chronic myositis (juvenile dermatomyositis and adult polymyositis). This may have been one of the first studies to provide solid evidence of the persistent presence of enterovirus in the tissues.
- Archard et al 1988: [86] may have been the first to find evidence for persistent enterovirus infection in the skeletal muscles of ME/CFS patients, using molecular hybridization testing to detect enteroviral RNA. They found enteroviral RNA even in ME/CFS patients who had had this illness for 20 years.
- Righthand et al 1989: [87] demonstrated that an echovirus 6 infection could be maintained for over 6 years in a cell line, and that this infection was and was not cytolytic (showed no cytopathic effect) when transfected into uninfected susceptible cells.
- Cunningham et al 1990: [88] was possibly the first indication that there was something odd about persistent enterovirus infections. In normal lytic enterovirus infections, there is around 100 times more positive strand RNA than negative strand RNA in infected cells. But in the enterovirus-infected muscles of ME/CFS patients, Cunningham et al found roughly equal amounts of positive and negative strand RNA. Thus they found instead of the normal approximately 100:1 positive to negative strand ratio, in ME/CFS the ratio is closer to 1:1. The authors concluded that this finding of near equal amounts, together with the failure to isolate and culture infectious lytic virus or detect virus-specific antigens, suggested that a mutant defective enterovirus may be responsible for these persistent muscle infections.
- Archard et al 1991: _last_=_Archard1991-89|[89] found a reduced positive to negative strand RNA ratio of around 1:1 in the heart muscle in dilated cardiomyopathy, and in myocarditis after the acute inflammation had cleared up. This is just as Cunningham had found in the skeletal muscles of ME/CFS patients. The authors concluded that the persisting enterovirus infection of the myocardium is probably underpinned by defective virus mutants which do not complete a productive replication cycle.
- Klingel et al 1992: [90] found the same reduced positive to negative strand ratio of around 1:1 in an immunocompetent mouse model of chronic myocarditis. They also noted a strong correlation, both spatial and temporal, between viral replication and formation of lesions in both the acute and chronic phase of infection.
- Tam and Messner 1999: [39] in a chronic CVB myositis mouse model, detected a decreased positive to negative strand RNA ratio of about 1:1 in the muscle tissues, mirroring what Cunningham had found in ME/CFS patients. They also demonstrated that enteroviral persistence in the muscle is characterized by the formation of enteroviral dsRNA. The authors pointed out that intracellular ssRNA decays within hours, so they postulated that the persistence of non-cytolytic enterovirus may instead arise from the dsRNA component of this infection, suggesting that the stability of dsRNA prevents its elimination by the immune system. They also suggested this dsRNA may contribute to pathogenicity of non-cytolytic enterovirus infection. So in the Tam and Messner model of non-cytolytic enterovirus, the viral dsRNA is hypothesized to be central to sustaining this form of infection.
- Feuer et al 2002: [91] proposed that persistent coxsackievirus B infection may be based on infection of quiescent cells, and observed that in quiescent cells, CVB appears to be able to persist in a form similar to viral latency.
- Kim et al 2005: [30] in this landmark study, Prof Nora Chapman identified the mechanism which underpins enteroviral RNA persistence. They isolated persistent coxsackievirus B3 from the hearts of mice that had been infected with this enterovirus one year earlier, and discovered mutations in this virus: deletions in the 5′ region at the end of the viral genome. They point out that such deletions are observed to inhibit lytic virus replication by decreasing positive strand viral RNA production, and propose that this lytic virus inhibition provides the opportunity for mutated virus populations to dominate the cell, thereby giving birth to persistent non-cytolytic infection. In the Chapman theory of non-cytolytic enterovirus, the deletions in the viral genome are central to creating and sustaining this persistent form of enterovirus infection.
- Chapman et al 2008: [1] found for the first time non-cytolytic enterovirus with deletions in the genomic 5′ region in human coxsackievirus B2 myocarditis. Previously non-cytolytic enterovirus with such deletions had only been detected in mouse models.
- Chia and Chia 2008: [33] found enteroviral VP1 protein in stomach tissues of 82% of ME/CFS patients, compared to 20% of the controls, and found enteroviral RNA in 37% of patient tissues compared to 5% of controls. They discovered that in the stomach tissues of ME/CFS patients, lytic virus is not always found, despite the presence of enteroviral RNA. This is a signature of non-cytolytic infection.
- Feuer et al 2009: [92] showed that coxsackievirus B3 may persist as a chronic low-level non-cytolytic infection in the central nervous system of mice. Three case studies have also found chronic enterovirus infection in the brains of ME/CFS patients; see post-mortem brain studies.
- Lévêque et al 2012: [93] found for the first time non-cytolytic enterovirus in the heart tissues in human idiopathic dilated cardiomyopathy: they found enterovirus genomes with deletions in the 5′ region, and they found a reduced positive to negative enteroviral ssRNA ratio.
- Tracy et al 2015: [18] found non-cytolytic coxsackievirus B with deletions in the 5′ region can persist in the pancreas of mice. This has implications in the study of type 1 diabetes, which has long been linked to CVB. The fact that non-cytolytic CVB can form long-term infection in the pancreas raises the possibility that this infection might be a cause of TID, at least in a subset of patients. Pre-existing insulitis in murine pancreatic islets makes the islets more susceptible to CVB infection.[94][95]
- Chia et al 2015: [25] using VP1 and dsRNA staining found both enteroviral VP1 protein and enteroviral dsRNA in the stomach tissues of functional dyspepsia and chronic gastritis patients both with and without an ME/CFS diagnosis. The dsRNA is postulated to be a mechanism of non-cytolytic enteroviral persistence.
- Smithee 2015: [96] demonstrated that artificially engineered non-cytolytic CVB populations in vitro displayed impaired replication, with RNA levels 100,000-fold lower than those of lytic (wild-type) virus.
- Bouin et al 2016: [11] found a mix of non-cytolytic (terminally deleted) and complete coxsackievirus B3 genomes in the endomyocardial tissues of a patient with dilated cardiomyopathy, along with a low viral RNA load, and a decreased positive to negative strand RNA ratio of 4.8:1. They found the majority of the CVB genomes in the tissues were terminally deleted (with deletions varying from 15 to 48 nucleotides is length), but a small minority (0.9%) of these genomes were found to be the complete genomes of the lytic (wild-type) virus.
- Lévêque et al 2017: [97] in a series of in vitro experiments on non-cytolytic coxsackievirus B3 with artificially engineered 5′ terminal deletions, found that this terminally deleted CVB3 RNA synthesized viral proteins in similar quantities to lytic CVB3 infection, although non-cytolytic CVB3 RNA with the largest deletions generated less protein (see Fig 4). They were unable to detect RNA synthesis in CVB3 with deletions larger than 21 nucleotides, though point out that other studies have detected very low levels of RNA replication in such terminally deleted enteroviruses in vitro. They suggest this discrepancy is most likely be due to their testing methods being not sufficiently sensitive to detect the very low levels of RNA present. They also suggest another possibility for this discrepancy: that the small amount of lytic virus which Bouin et al found in vivo mixed in with the terminally deleted virus could act as a helper virus, facilitating terminally deleted viruses to replicate. In this model, non-cytolytic (terminally deleted) viruses must coexist with a small amount of lytic virus in order to facilitate their replication (or facilitate more efficient replication).
- Flynn et al 2017: [98] argue that the persistent enteroviral RNA found in myocarditis and dilated cardiomyopathy are probably not the cause of these diseases. They point out that in the experiments of Nora Chapman and colleagues inoculating mice with cultured non-cytolytic infection, this infection made its way to the heart, where it persisted, but it did not trigger any detectable pathology. However, Nora Chapman says it likely requires a high level of acute infection to seed a sufficient number of quiescent cells with enterovirus before a non-cytolytic infection emerges.[99]
Note on defective interfering viruses
Non-cytolytic enteroviruses are similar to defective interfering (DI) viruses: both have deletions in their genomes. However, one difference is that DI viruses cannot replicate on their own, they are only able to replicate with the help of the lytic virus with a complete genome,[100] whereas non-cytolytic enteroviruses are thought to be able to replicate independently.
Although true independent replication is not entirely certain, as in a non-cytolytic enterovirus infection, Bouin et al[11] found most of the viral genomes are defective due to deletions, but they also found 0.9% of the genomes to be those of the intact lytic virus. Thus the lytic virus genome is still present in small quantities in non-cytolytic infection, and Lévêque[97] suggests that this small amount of lytic virus RNA in the cell might be helping the defective viruses to replicate. However, this low level of lytic RNA found in non-cytolytic infection is not the same as an acute lytic infection, so in that respect, it is clear that non-cytolytic enterovirus can replicate independently to the lytic virus and its lifecycle.
Another difference is that defective interfering viruses have large genome deletions, typically consisting of only 5-10% of the genome of the parental lytic virus (though in the case of picornavirus DI viruses, these usually comprise 85% or more of the parental genome).[101] By contrast, in the case of the genomic deletions of non-cytolytic enterovirus, less than 0.7% of the parental genome is lost (non-cytolytic enterovirus deletions are 7 to 49 nucleotides in size,[30] and the full coxsackievirus B genome comprises around 7,400 nucleotides).[42] So non-cytolytic enterovirus still possesses most of the parental lytic virus genome, thus retaining most of the functionality.
See also
- Enterovirus
- Coxsackie B virus
- Echovirus
- Viral testing in ME/CFS
- List of enterovirus infection studies
- Epidemic myalgic encephalomyelitis
- Post-mortem brain studies
- Nora Chapman
- John Chia
Learn more
How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Presentation by Prof Nora M. Chapman.[102]
Invest in ME International ME Conference, London 2010 Persistent Enteroviral Infections. Presentation by Prof Nora M. Chapman.[103]
Human Enterovirus Persistence: Potential for Exacerbation of Myocarditis. Talk by Prof Nora M. Chapman.
Human Enteroviruses and Chronic Infectious Disease. Steven Tracy & Nora Chapman. Also as (p.23-31).[104]
The role of enterovirus in chronic fatigue syndrome. Dr John Chia.[82]
Replication Defective Enterovirus Infections: Implications for Type I Diabetes. Presentation slides by Nora M. Chapman.
Human Enteroviruses and Type 1 Diabetes (p.27-33). Prof Steven Tracy. Also as (p.27-33).[95]
Revealing enterovirus infection in chronic human disorders: An integrated diagnostic approach.[66]
Enterovirus Persistence as a Mechanism in the Pathogenesis of Type 1 Diabetes.
Persistent Enterovirus Infection: Little Deletions, Long Infections. Review paper by Prof Nora M. Chapman, 2022.
References
- ↑ 1.0 1.1 1.2 1.3 Chapman, Nora M.; Kim, Kyung-Soo; Drescher, Kristen M.; Oka, Kuniyuki; Tracy, Steven (June 5, 2008). "5' terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart". Virology. 375 (2): 480–491. doi:10.1016/j.virol.2008.02.030. ISSN 0042-6822. PMC 2440640. PMID 18378272.
- ↑ Chapman, N.M.; Kim, K.S. (2008). "Persistent coxsackievirus infection: enterovirus persistence in chronic myocarditis and dilated cardiomyopathy". Current Topics in Microbiology and Immunology. 323: 275–292. ISSN 0070-217X. PMID 18357775.
- ↑ 3.0 3.1 3.2 Archard, L. C.; Richardson, P.J.; Olsen, E.G.; Dubowitz, V.; Sewry, C.; Bowles, N.E. (1987). "The role of Coxsackie B viruses in the pathogenesis of myocarditis, dilated cardiomyopathy and inflammatory muscle disease". Biochemical Society Symposium. 53: 51–62. ISSN 0067-8694. PMID 2847741.
despite many attempts, isolation of infectious virus or immunofluorescent detection of virus-specific antigens in the affected tissue is rare
- ↑ Bowles, N. E.; Rose, M.L.; Taylor, P.; Banner, N.R.; Morgan-Capner, P.; Cunningham, L.; Archard, L.C.; Yacoub, M.H. (November 1989). "End-stage dilated cardiomyopathy. Persistence of enterovirus RNA in myocardium at cardiac transplantation and lack of immune response". Circulation. 80 (5): 1128–1136. ISSN 0009-7322. PMID 2553297.
- ↑ Andreoletti, L.; Wattre, P.; Decoene, C.; Lobert, P.E.; Dewilde, A.; Hober, D. (November 1995). "Detection of enterovirus-specific RNA sequences in explanted myocardium biopsy specimens from patients with dilated or ischemic cardiomyopathy". Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 21 (5): 1315–1317. ISSN 1058-4838. PMID 8589166.
- ↑ Andreoletti, L.; Hober, D.; Decoene, C.; Copin, M.C.; Lobert, P.E.; Dewilde, A.; Stankowiac, C.; Wattre, P. (January 1996). "Detection of enteroviral RNA by polymerase chain reaction in endomyocardial tissue of patients with chronic cardiac diseases". Journal of Medical Virology. 48 (1): 53–59. doi:10.1002/(SICI)1096-9071(199601)48:13.0.CO;2-K. ISSN 0146-6615. PMID 8825711.
- ↑ Li, Y.; Bourlet, T.; Andreoletti, L.; Mosnier, J.F.; Peng, T.; Yang, Y.; Archard, L.C.; Pozzetto, B.; Zhang, H. (January 25, 2000). "Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy". Circulation. 101 (3): 231–234. ISSN 1524-4539. PMID 10645916.
- ↑ Andréoletti, L.; Bourlet, T.; Moukassa, D.; Rey, L.; Hot, D.; Li, Y.; Lambert, V.; Gosselin, B.; Mosnier, J.F. (October 2000). "Enteroviruses can persist with or without active viral replication in cardiac tissue of patients with end-stage ischemic or dilated cardiomyopathy". The Journal of Infectious Diseases. 182 (4): 1222–1227. doi:10.1086/315818. ISSN 0022-1899. PMID 10979922.
- ↑ 9.0 9.1 Rey, L.; Lambert, V.; Wattré, P.; Andréoletti, L. (June 2001). "Detection of enteroviruses ribonucleic acid sequences in endomyocardial tissue from adult patients with chronic dilated cardiomyopathy by a rapid RT-PCR and hybridization assay". Journal of Medical Virology. 64 (2): 133–140. ISSN 0146-6615. PMID 11360245.
Of the 55 biopsy specimens aseptically collected from the explanted hearts of 55 patients, 21 (38.2%) were positive by RT-PCR microplate assay, whereas only 19 (34.5%) were positive by nested RT-PCR assay and none were positive by classical cell culture assays.
- ↑ Nguyen, Yohan; Renois, Fanny; Leveque, Nicolas; Giusti, Delphine; Picard-Maureau, Marcus; Bruneval, Patrick; Fornes, Paul; Andreoletti, Laurent (July 2013). "Virus detection and semiquantitation in explanted heart tissues of idiopathic dilated cardiomyopathy adult patients by use of PCR coupled with mass spectrometry analysis". Journal of Clinical Microbiology. 51 (7): 2288–2294. doi:10.1128/JCM.00820-13. ISSN 1098-660X. PMC 3697695. PMID 23658274.
- ↑ 11.0 11.1 11.2 Bouin, Alexis; Nguyen, Yohan; Wehbe, Michel; Renois, Fanny; Fornes, Paul; Bani-Sadr, Firouze; Metz, Damien; Andreoletti, Laurent (August 2016). "Major Persistent 5' Terminally Deleted Coxsackievirus B3 Populations in Human Endomyocardial Tissues". Emerging Infectious Diseases. 22 (8): 1488–1490. doi:10.3201/eid2208.160186. ISSN 1080-6059. PMC 4982168. PMID 27434549.
Our results estimated a major proportion (84.8%) of reads presenting with a terminal 48-nt deletion associated with minor proportions of reads deleted of 15 nt (14.3%) and nondeleted (0.9%).
- ↑ Vandenberghe, Nadia; Leveque, Nicolas; Corcia, Philippe; Brunaud-Danel, Véronique; Salort-Campana, Emmanuelle; Besson, Gérard; Tranchant, Christine; Clavelou, Pierre; Beaulieux, Frédérique (May 3, 2010). "Cerebrospinal fluid detection of enterovirus genome in ALS: a study of 242 patients and 354 controls". Amyotrophic Lateral Sclerosis: Official Publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 11 (3): 277–282. doi:10.3109/17482960903262083. ISSN 1471-180X. PMID 19900148.
- ↑ Woodall, C. J.; Riding, M.H.; Graham, D.I.; Clements, G.B. (June 11, 1994). "Sequences specific for enterovirus detected in spinal cord from patients with motor neurone disease". BMJ (Clinical research ed.). 308 (6943): 1541–1543. ISSN 0959-8138. PMC 2540520. PMID 8019310.
- ↑ Xue, Yuan Chao; Feuer, Ralph; Cashman, Neil; Luo, Honglin (2018). "Enteroviral Infection: The Forgotten Link to Amyotrophic Lateral Sclerosis?". Frontiers in Molecular Neuroscience. 11. doi:10.3389/fnmol.2018.00063. ISSN 1662-5099. PMC 5857577. PMID 29593492.
- ↑ Dourmashkin, Robert R.; McCall, Sherman A.; Dourmashkin, Neil; Hannah, Matthew J. (2018). "Virus-like particles and enterovirus antigen found in the brainstem neurons of Parkinson's disease". F1000Research. 7: 302. doi:10.12688/f1000research.13626.2. ISSN 2046-1402. PMC 5968367. PMID 29899977.
- ↑ Triantafyllopoulou, Antigoni; Tapinos, Nikos; Moutsopoulos, Haralampos M. (September 2004). "Evidence for coxsackievirus infection in primary Sjögren's syndrome". Arthritis and Rheumatism. 50 (9): 2897–2902. doi:10.1002/art.20463. ISSN 0004-3591. PMID 15457458.
- ↑ Muir, P.; Nicholson, F.; Sharief, M.K.; Thompson, E.J.; Cairns, N.J.; Lantos, P.; Spencer, G.T.; Kaminski, H.J.; Banatvala, J.E. (May 25, 1995). "Evidence for persistent enterovirus infection of the central nervous system in patients with previous paralytic poliomyelitis". Annals of the New York Academy of Sciences. 753: 219–232. ISSN 0077-8923. PMID 7611631.
- ↑ 18.0 18.1 Tracy, Steven; Smithee, Shane; Alhazmi, Abdulaziz; Chapman, Nora (February 2015). "Coxsackievirus can persist in murine pancreas by deletion of 5' terminal genomic sequences". Journal of Medical Virology. 87 (2): 240–247. doi:10.1002/jmv.24039. ISSN 1096-9071. PMID 25111164.
- ↑ Chehadeh, W.; Kerr-Conte, J.; Pattou, F.; Alm, G.; Lefebvre, J.; Wattré, P.; Hober, D. (November 2000). "Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells". Journal of Virology. 74 (21): 10153–10164. ISSN 0022-538X. PMID 11024144.
- ↑ Krogvold, Lars; Edwin, Bjørn; Buanes, Trond; Frisk, Gun; Skog, Oskar; Anagandula, Mahesh; Korsgren, Olle; Undlien, Dag; Eike, Morten C. (May 2015). "Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes". Diabetes. 64 (5): 1682–1687. doi:10.2337/db14-1370. ISSN 1939-327X. PMID 25422108.
- ↑ Nyström, Niklas; Berg, Tove; Lundin, Elin; Skog, Oskar; Hansson, Inga; Frisk, Gun; Juko-Pecirep, Ivana; Nilsson, Mats; Gyllensten, Ulf (June 27, 2013). "Human enterovirus species B in ileocecal Crohn's disease". Clinical and Translational Gastroenterology. 4: e38. doi:10.1038/ctg.2013.7. ISSN 2155-384X. PMC 3696939. PMID 23804031.
- ↑ Burch, G. E.; Sun, S.C.; Colcolough, H.L.; Sohal, R.S.; DePasquale, N.P. (July 1967). "Coxsackie B viral myocarditis and valvulitis identified in routine autopsy specimens by immunofluorescent techniques". American Heart Journal. 74 (1): 13–23. ISSN 0002-8703. PMID 5338726.
- ↑ Li, Y.; Pan, Z.; Ji, Y.; Peng, T.; Archard, L.C.; Zhang, H. (April 1, 2002). "Enterovirus replication in valvular tissue from patients with chronic rheumatic heart disease". European Heart Journal. 23 (7): 567–573. doi:10.1053/euhj.2001.2837. ISSN 0195-668X.
- ↑ Kim, Julian K. S.; Zhu, Zhen; Casale, George; Koutakis, Panagiotis; McComb, Rodney D.; Swanson, Stanley; Thompson, Jonathan; Miserlis, Dimitrios; Johanning, Jason M. (August 6, 2013). "Human enterovirus in the gastrocnemius of patients with peripheral arterial disease". Journal of the American Heart Association. 2 (4): e000082. doi:10.1161/JAHA.113.000082. ISSN 2047-9980. PMC 3828788. PMID 23920231.
- ↑ 25.0 25.1 25.2 Chia, John K.; Chia, Andrew Y.; Wang, David; El-Habbal, Rabiha (2015). "Functional Dyspepsia and Chronic Gastritis Associated with Enteroviruses". Open Journal of Gastroenterology. 05 (04): 21–27. doi:10.4236/ojgas.2015.54005. ISSN 2163-9450.
- ↑ 26.0 26.1 Kim, K.-S.; Tracy, S.; Tapprich, W.; Bailey, J.; Lee, C.-K.; Kim, K.; Barry, W.H.; Chapman, N.M. (June 2005). "5'-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA". Journal of Virology. 79 (11): 7024–7041. doi:10.1128/JVI.79.11.7024-7041.2005. ISSN 0022-538X. PMC 1112132. PMID 15890942.
Picornavirus infections are generally considered to be acute and cleared rapidly by the host adaptive immune response.
- ↑ 27.0 27.1 Flynn, Claudia T.; Kimura, Taishi; Frimpong-Boateng, Kwesi; Harkins, Stephanie; Whitton, J. Lindsay (December 2017). "Immunological and pathological consequences of coxsackievirus RNA persistence in the heart". Virology. 512: 104–112. doi:10.1016/j.virol.2017.09.017. ISSN 0042-6822. PMC 5653433. PMID 28950225.
until relatively recently, enteroviruses were thought to cause only acute infections, and to be completely eradicated with ~ 2 weeks of the primary infection.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode 10:10". YouTube.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode 3:11". YouTube.
- ↑ 30.0 30.1 30.2 30.3 30.4 Kim, K.-S.; Tracy, S.; Tapprich, W.; Bailey, J.; Lee, C.-K.; Kim, K.; Barry, W.H.; Chapman, N.M. (June 2005). "5'-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA". Journal of Virology. 79 (11): 7024–7041. doi:10.1128/JVI.79.11.7024-7041.2005. ISSN 0022-538X. PMC 1112132. PMID 15890942.
These results represent the first report of an in vivo evolution of a wild-type enterovirus population into a quasispecies that has 5′-terminal genomic deletions ranging from 7 to 49 nucleotides.
- ↑ Tam, P. E.; Messner, R.P. (December 1999). "Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution". Journal of Virology. 73 (12): 10113–10121. ISSN 0022-538X. PMID 10559326.
By 1 month after infection, which is a time when infectious virus can no longer be recovered, the level of plus-strand RNA in muscle had diminished to a fourfold excess over minus-strand RNA. Given the slightly higher efficiency for RT-PCR of the plus-strand RNA, actual levels of plus- and minus-strand RNAs were nearly equal. Moreover, the RNA appeared to persist as a double-stranded complex, and absolute levels of minus-strand RNA were similar to those which were present during acute infection.
- ↑ Cunningham, L.; Bowles, N.E.; Archard, L.C. (October 1991). "Persistent virus infection of muscle in postviral fatigue syndrome". British Medical Bulletin. 47 (4): 852–871. ISSN 0007-1420. PMID 1665379.
Attempts to demonstrate either infectious virus or virus-specific antigens in muscle samples from PVFS patients had been consistently unsuccessful.
- ↑ 33.0 33.1 33.2 Chia, J.K. S.; Chia, A.Y. (January 2008). "Chronic fatigue syndrome is associated with chronic enterovirus infection of the stomach". Journal of Clinical Pathology. 61 (1): 43–48. doi:10.1136/jcp.2007.050054. ISSN 1472-4146. PMID 17872383.
No significant cytopathic effect was shown in the EV RNA-positive cultures, whereas wild type strains of enteroviruses would cause major cytopathic changes within one week.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode 6:57". YouTube.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode 13:46". YouTube.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 13:46". YouTube.
- ↑ Lévêque, Nicolas; Garcia, Magali; Bouin, Alexis; Nguyen, Joseph H.C.; Tran, Genevieve P.; Andreoletti, Laurent; Semler, Bert L. (August 15, 2017). "Functional Consequences of RNA 5'-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation". Journal of Virology. 91 (16). doi:10.1128/JVI.00423-17. ISSN 1098-5514. PMC 5533909. PMID 28539455.
However, the molecular mechanisms by which the virus can persist in the heart from acute myocarditis to DCM are poorly understood, limiting the development of new specific therapeutic strategies.
- ↑ Chia, J.K.S. (November 2005). "The role of enterovirus in chronic fatigue syndrome". Journal of Clinical Pathology. 58 (11): 1126–1132. doi:10.1136/jcp.2004.020255. ISSN 0021-9746. PMC 1770761. PMID 16254097.
However, enough positive and negative strands probably recombine to form stable double stranded RNAs, which are resistant to RNAse L inactivation, and the life cycle will start again when the pressure of the immune response decreases.
- ↑ 39.0 39.1 39.2 Tam, P. E.; Messner, R.P. (December 1999). "Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution". Journal of Virology. 73 (12): 10113–10121. ISSN 0022-538X. PMID 10559326.
Since single-stranded intracellular viral RNAs decay within hours, this double-stranded form may lend stability to and protect the RNA from degradation, thereby promoting long-term persistence.
- ↑ Chia, J.K.S. (November 2005). "The role of enterovirus in chronic fatigue syndrome". Journal of Clinical Pathology. 58 (11): 1126–1132. doi:10.1136/jcp.2004.020255. ISSN 0021-9746. PMC 1770761. PMID 16254097.
It is probable that viral RNA found inside cells, in a stable double stranded form, can dissociate and replicate using viral RNA replicase
- ↑ Chia, John (2010). "Enterovirus Infection in ME/CFS. Presentation at the Invest in ME International ME Conference, London 2010 (available on DVD). Timecode: 42:36". YouTube.
- ↑ 42.0 42.1 42.2 42.3 Lévêque, Nicolas; Garcia, Magali; Bouin, Alexis; Nguyen, Joseph H.C.; Tran, Genevieve P.; Andreoletti, Laurent; Semler, Bert L. (August 15, 2017). "Functional Consequences of RNA 5'-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation". Journal of Virology. 91 (16). doi:10.1128/JVI.00423-17. ISSN 1098-5514. PMC 5533909. PMID 28539455.
Interestingly, Hyde and colleagues demonstrated that alphaviruses, which are also single-stranded, positive-strand RNA viruses, use mutations within the 5′ noncoding region affecting secondary-structural elements of their RNAs to alter interferon-stimulated protein binding and functions. Their results suggest an evasion mechanism by which a deleted virus with modified 5′ RNA secondary structures could avoid immune restriction, despite type 1 IFN secretion and interferon-stimulated gene transcription, leading to long-term virus persistence in the heart.
- ↑ Hyde, Jennifer L.; Gardner, Christina L.; Kimura, Taishi; White, James P.; Liu, Gai; Trobaugh, Derek W.; Huang, Cheng; Tonelli, Marco; Paessler, Slobodan (February 14, 2014). "A viral RNA structural element alters host recognition of non-self RNA". Science (New York, N.Y.). 343 (6172): 783–787. doi:10.1126/science.1248465. ISSN 0036-8075. PMC 4209899. PMID 24482115.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 13:15". YouTube.
- ↑ Xiong, Dingding; Yajima, Toshitaka; Lim, Byung-Kwan; Stenbit, Antine; Dublin, Andrew; Dalton, Nancy D.; Summers-Torres, Daphne; Molkentin, Jeffery D.; Duplain, Herve (January 2, 2007). "Inducible cardiac-restricted expression of enteroviral protease 2A is sufficient to induce dilated cardiomyopathy". Circulation. 115 (1): 94–102. doi:10.1161/CIRCULATIONAHA.106.631093. ISSN 1524-4539. PMID 17190866.
- ↑ Lévêque, Nicolas; Garcia, Magali; Bouin, Alexis; Nguyen, Joseph H.C.; Tran, Genevieve P.; Andreoletti, Laurent; Semler, Bert L. (August 15, 2017). "Functional Consequences of RNA 5'-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation". Journal of Virology. 91 (16). doi:10.1128/JVI.00423-17. ISSN 1098-5514. PMC 5533909. PMID 28539455.
Expression of 2A can cause significant impairment of cardiomyocyte function through proteolytic cleavage of dystrophin, resulting in a decrease in cell contractility, an increase in membrane permeability, and a focal spread of the virus to adjacent cells.
- ↑ Zhang, H.; Li, Y.; Peng, T.; Aasa, M.; Zhang, L.; Yang, Y.; Archard, L.C. (May 2000). "Localization of enteroviral antigen in myocardium and other tissues from patients with heart muscle disease by an improved immunohistochemical technique". The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 48 (5): 579–584. doi:10.1177/002215540004800501. ISSN 0022-1554. PMID 10769041.
enteroviral capsid protein VP1 was detected in duplicate myocardial tissue sections from six of nine myocarditis and three of four DCM cases.
- ↑ Lévêque, Nicolas; Garcia, Magali; Bouin, Alexis; Nguyen, Joseph H.C.; Tran, Genevieve P.; Andreoletti, Laurent; Semler, Bert L. (July 27, 2017). "Functional Consequences of RNA 5′-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation". Journal of Virology. 91 (16). doi:10.1128/JVI.00423-17. ISSN 0022-538X. PMC 5533909. PMID 28539455.
Overall, the data displayed in Fig. 4 show similar patterns of translation products for all RNAs tested, although the RNAs with the largest deletions (TD30 and TD49) produced lower overall levels of CVB3 proteins, particularly at the lower concentrations of RNA.
- ↑ Chia, J.K.S. (November 2005). "The role of enterovirus in chronic fatigue syndrome". Journal of Clinical Pathology. 58 (11): 1126–1132. doi:10.1136/jcp.2004.020255. ISSN 0021-9746. PMC 1770761. PMID 16254097.
Among other immunostimulatory effects, double stranded RNA is a potent inducer of interferon synthesis, which activates intracellular RNase, with resultant degradation of excessive single stranded RNA. The finding of a higher level of RNase L activity in the mononuclear cells of patients with CFS is consistent with this paradigm.
- ↑ Lévêque, Nicolas; Garcia, Magali; Bouin, Alexis; Nguyen, Joseph H.C.; Tran, Genevieve P.; Andreoletti, Laurent; Semler, Bert L. (August 15, 2017). "Functional Consequences of RNA 5'-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation". Journal of Virology. 91 (16). doi:10.1128/JVI.00423-17. ISSN 1098-5514. PMC 5533909. PMID 28539455.
The synthesis of even low levels of double-stranded RNA by TD viruses could be sufficient to induce type I interferon (IFN) secretion by infected cardiomyocytes. ... It remains to be seen if innate immune sensors in cardiomyocytes can detect the very low levels of dsRNA synthesized by terminally deleted viruses and subsequently stimulate type I interferon secretion.
- ↑ Flynn, Claudia T.; Kimura, Taishi; Frimpong-Boateng, Kwesi; Harkins, Stephanie; Whitton, J. Lindsay (December 2017). "Immunological and pathological consequences of coxsackievirus RNA persistence in the heart". Virology. 512: 104–112. doi:10.1016/j.virol.2017.09.017. ISSN 0042-6822. PMC 5653433. PMID 28950225.
so we next used PCR arrays to evaluate the expression of host interferon-related mRNAs. No significant IFN signature was detected in RNA+ mice ... In summary, our data suggest that persistent cardiac CVB3 RNA does not cause a substantial ongoing innate response in the heart ... We therefore propose that the viral RNAs that remain at 30d p.i. most probably play no role in ongoing pathogenesis, and are merely the last remnants of the preceding infection.
- ↑ Schulze, K.; Witzenbichler, B.; Christmann, C.; Schultheiss, H.P. (October 1999). "Disturbance of myocardial energy metabolism in experimental virus myocarditis by antibodies against the adenine nucleotide translocator". Cardiovascular Research. 44 (1): 91–100. ISSN 0008-6363. PMID 10615393.
- ↑ Schultheiss, H. P.; Schulze, K.; Dörner, A. (October 1996). "Significance of the adenine nucleotide translocator in the pathogenesis of viral heart disease". Molecular and Cellular Biochemistry (163–164): 319–327. ISSN 0300-8177. PMID 8974071.
- ↑ Schulze, K.; Schultheiss, H.P. (December 1995). "The role of the ADP/ATP carrier in the pathogenesis of viral heart disease". European Heart Journal. 16 (Suppl O): 64–67. ISSN 0195-668X. PMID 8682105.
- ↑ Schultheiss, H.P. (May 1993). "Disturbance of the myocardial energy metabolism in dilated cardiomyopathy due to autoimmunological mechanisms". Circulation. 87 (5 Suppl): IV43–48. ISSN 0009-7322. PMID 8485833.
- ↑ Schulze, K.; Becker, B.F.; Schauer, R.; Schultheiss, H.P. (March 1990). "Antibodies to ADP-ATP carrier--an autoantigen in myocarditis and dilated cardiomyopathy--impair cardiac function". Circulation. 81 (3): 959–969. ISSN 0009-7322. PMID 2155073.
- ↑ 57.0 57.1 57.2 Kim, K.-S.; Tracy, S.; Tapprich, W.; Bailey, J.; Lee, C.-K.; Kim, K.; Barry, W.H.; Chapman, N.M. (June 2005). "5'-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA". Journal of Virology. 79 (11): 7024–7041. doi:10.1128/JVI.79.11.7024-7041.2005. ISSN 0022-538X. PMC 1112132. PMID 15890942.
The detection of the largest capsid protein, VP1, by Western blot analysis in cells inoculated with CVB3/TD8 and TD50, together with the ablation of detectable CVB3/TD RNA in cells infected with virus in the presence of anti-CVB3 neutralizing antibody, strongly suggests that CVB3/TD strains are normally encapsidated and transmitted.
- ↑ Lévêque, Nicolas; Garcia, Magali; Bouin, Alexis; Nguyen, Joseph H.C.; Tran, Genevieve P.; Andreoletti, Laurent; Semler, Bert L. (August 15, 2017). "Functional Consequences of RNA 5'-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation". Journal of Virology. 91 (16). doi:10.1128/JVI.00423-17. ISSN 1098-5514. PMC 5533909. PMID 28539455.
It is known that the host protein hnRNP C can bind both the 3′ and 5′ termini of poliovirus negative-strand RNA intermediates, an interaction proposed to promote the synthesis of positive-strand enterovirus RNA molecules. Thus, the 5′-terminal deletions of positive-strand RNAs would lead to deletions in the 3′ ends of negative-strand RNAs, possibly reducing the binding of hnRNP C to these replication intermediates. Loss of such binding might also contribute to the reduction of positive-strand RNA synthesis observed with TD viruses.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 4:00". YouTube.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 5:38". YouTube.
- ↑ Lévêque, Nicolas; Garcia, Magali; Bouin, Alexis; Nguyen, Joseph H.C.; Tran, Genevieve P.; Andreoletti, Laurent; Semler, Bert L. (August 15, 2017). "Functional Consequences of RNA 5'-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation". Journal of Virology. 91 (16). doi:10.1128/JVI.00423-17. ISSN 1098-5514. PMC 5533909. PMID 28539455.
Moreover, low levels of hnRNP C expression in the cytoplasm of quiescent and differentiated cells (like cardiomyocytes) have been suggested to give putative hnRNP C-independent TD genomes an evolutionary advantage over hnRNP C-dependent wild-type enterovirus RNA, thereby enabling them to become the dominant virus population in persistent infections.
- ↑ Bouin, Alexis; Nguyen, Yohan; Wehbe, Michel; Renois, Fanny; Fornes, Paul; Bani-Sadr, Firouze; Metz, Damien; Andreoletti, Laurent (August 2016). "Major Persistent 5' Terminally Deleted Coxsackievirus B3 Populations in Human Endomyocardial Tissues". Emerging Infectious Diseases. 22 (8): 1488–1490. doi:10.3201/eid2208.160186. ISSN 1080-6059. PMC 4982168. PMID 27434549.
- ↑ Kim, K.-S.; Chapman, N.M.; Tracy, S. (February 2008). "Replication of coxsackievirus B3 in primary cell cultures generates novel viral genome deletions". Journal of Virology. 82 (4): 2033–2037. doi:10.1128/JVI.01774-07. ISSN 1098-5514. PMC 2258713. PMID 18057248.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 13:53". YouTube.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 7:57". YouTube.
- ↑ 66.0 66.1 Genoni, Angelo; Canducci, Filippo; Rossi, Agostino; Broccolo, Francesco; Chumakov, Konstantin; Bono, Giorgio; Salerno-Uriarte, Jorge; Salvatoni, Alessandro; Pugliese, Alberto (July 10, 2017). "Revealing enterovirus infection in chronic human disorders: An integrated diagnostic approach". Scientific Reports. 7 (1). doi:10.1038/s41598-017-04993-y. ISSN 2045-2322. PMC 5504018. PMID 28694527.
In persistently infected cultures, virus transmission may occur through the release of extracellular vesicles, formation of cell protrusions and intercellular bridges. This exit mode may facilitate virus dissemination in vivo in the presence of a robust immune response.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 6:57". YouTube.
- ↑ Chapman, Nora (2012). "Replication Defective Enterovirus Infections:Implications for Type I Diabetes. Slides from a presentation by Prof Nora M. Chapman given at the 2012 Annual Meeting of the Network for Pancreatic Organ Donors with Diabetes, Page 22" (PDF). jdrfnpod.
- ↑ Christensen, M. L.; Pachman, L.M.; Schneiderman, R.; Patel, D.C.; Friedman, J.M. (November 1986). "Prevalence of Coxsackie B virus antibodies in patients with juvenile dermatomyositis". Arthritis and Rheumatism. 29 (11): 1365–1370. ISSN 0004-3591. PMID 3022759.
Patient and control sera were tested by 2 main serologic methods that primarily reflect IgG function: the complement-fixation (CF) test and the neutralization test. The CF test measures antibody that, usually, can be detected for only a few months after the onset of a viral infection, whereas neutralizing antibody can be detected for years after the initial infection.
- ↑ Chia, John (2009). "Diagnosis and Treatment of Myalgic Encephalomyelitis / Chronic Fatigue Syndrome Associated with Chronic Enterovirus Infection. Presentation at the Invest in ME International ME Conference, London 2009 (available on DVD). Timecode: 27:51". Invest in ME Research.
The typical antibody that the laboratory would do is called the complement fixation test, which is neither sensitive nor specific. That means if you get a positive test, it's worthless. And if you get a negative test, it's worthless. Well that's wonderful.
- ↑ Chia, John (2008). "The Role of Enteroviruses in Myalgic Encephalomyelitis / Chronic Fatigue Syndrome. Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 10:34 ". YouTube.
- ↑ Chia, J K S (November 2005). "The role of enterovirus in chronic fatigue syndrome". Journal of Clinical Pathology. 58 (11): 1126–1132. doi:10.1136/jcp.2004.020255. ISSN 0021-9746. PMC 1770761. PMID 16254097.
During our study to determine the infectious aetiology in the first 200 patients with severe fatigue following flu-like illnesses, ... Significantly raised neutralising antibody titres against six coxsackieviruses or five echoviruses were found in more than half of those patients who had initial respiratory and/or gastrointestinal tract infections, compared with 150 control subjects
- ↑ Cambridge, G.; MacArthur, C.G.; Waterson, A.P.; Goodwin, J.F.; Oakley, C.M. (June 1979). "Antibodies to Coxsackie B viruses in congestive cardiomyopathy". British Heart Journal. 41 (6): 692–696. ISSN 0007-0769. PMID 223612.
High neutralisation titres (≥1024) to Coxsackie B viruses were more common among the patients than among the controls. ... A micro-neutralisation test system was used for the Coxsackie B antibody studies
- ↑ Chia, John. "Enterovirus Foundation". Enterovirus Foundation.
- ↑ Chia, John (2008). "The Role of Enteroviruses in Myalgic Encephalomyelitis / Chronic Fatigue Syndrome. Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 15:22". YouTube.
- ↑ Chia, John (2011). "The Pathogenic Role of Enteroviruses in Myalgic Encephalomyelitis / Chronic Fatigue Syndrome. Presentation at the State of Knowledge Workshop on ME/CFS, National Institutes of Health, April 2011, Day 1, Part 1. Timecode: 9:25". YouTube.
- ↑ Chia, John (2008). "The Role of Enteroviruses in Myalgic Encephalomyelitis / Chronic Fatigue Syndrome. Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 16:04". YouTube.
- ↑ Chia, John (2015). "Dr John Chia: Enteroviruses and Myalgic Encephalomyelitis / Chronic Fatigue Syndrome: An Update on Pathogenesis. Presentation at the Invest in ME International ME Conference, London 2015 (available on DVD). Timecode: 12:00". Invest in ME Research.
- ↑ "Autopsy in Myalgic Encephalomyelitis". See the section "Chia 2015 study".
- ↑ Brook, M. G.; Bannister, B.A.; Weir, W.R. (September 1993). "Interferon-alpha therapy for patients with chronic fatigue syndrome". The Journal of Infectious Diseases. 168 (3): 791–792. ISSN 0022-1899. PMID 8354926.
- ↑ Chia, John; Chia, A.Y. (2004). "Ribavirin and interferon-α for the treatment of patients with chronic fatigue syndrome associated with persistent coxsackievirus B infection: A preliminary observation" (PDF). Journal of Applied Research. 4 (2): 286–292.
- ↑ 82.0 82.1 82.2 Chia, J.K.S. (November 2005). "The role of enterovirus in chronic fatigue syndrome". Journal of Clinical Pathology. 58 (11): 1126–1132. doi:10.1136/jcp.2004.020255. ISSN 0021-9746. PMC 1770761. PMID 16254097.
To develop inhibitors for viral RNA replicase, the main mechanism for RNA replication, which allows the persistence of the viral genome in infected cells.
- ↑ Chia, John (2015). "Enteroviruses and Myalgic Encephalomyelitis / Chronic Fatigue Syndrome: An Update on Pathogenesis. Presentation at the Invest in ME International ME Conference, London 2015 (available on DVD). Timecode: 3:10". YouTube.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 3:27". YouTube.
- ↑ Chapman, N.M.; Ramsingh, A.I.; Tracy, S. (1997). Genetics of Coxsackievirus Virulence. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 227–258. doi:10.1007/978-3-642-60687-8_11. ISBN 9783642645075.
- ↑ Archard, L. C.; Bowles, N.E.; Behan, P.O.; Bell, E.J.; Doyle, D. (June 1988). "Postviral fatigue syndrome: persistence of enterovirus RNA in muscle and elevated creatine kinase". Journal of the Royal Society of Medicine. 81 (6): 326–329. doi:10.1177/014107688808100608. ISSN 0141-0768. PMC 1291623. PMID 3404526.
- ↑ Righthand, V. F.; Blackburn, R.V. (December 1989). "Steady-state infection by echovirus 6 associated with nonlytic viral RNA and an unprocessed capsid polypeptide". Journal of Virology. 63 (12): 5268–5275. ISSN 0022-538X. PMID 2585604.
- ↑ Cunningham, L.; Bowles, N.E.; Lane, R.J.; Dubowitz, V.; Archard, L.C. (June 1990). "Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA". The Journal of General Virology. 71 (Pt 6): 1399–1402. doi:10.1099/0022-1317-71-6-1399. ISSN 0022-1317. PMID 2161907.
- _last_=_Archard1991_89-0|↑ Archard, L. C.; Bowles, N.E.; Cunningham, L.; Freeke, C.A.; Olsen, E.G.; Rose, M.L.; Meany, B.; Why, H.J.; Richardson, P.J. (August 1991). "Molecular probes for detection of persisting enterovirus infection of human heart and their prognostic value". European Heart Journal. 12 (Suppl D): 56–59. ISSN 0195-668X. PMID 1655452.
- ↑ Klingel, K.; Hohenadl, C.; Canu, A.; Albrecht, M.; Seemann, M.; Mall, G.; Kandolf, R. (January 1, 1992). "Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation". Proceedings of the National Academy of Sciences of the United States of America. 89 (1): 314–318. ISSN 0027-8424. PMID 1309611.
- ↑ Feuer, Ralph; Mena, Ignacio; Pagarigan, Robb; Slifka, Mark K.; Whitton, J. Lindsay (May 2002). "Cell cycle status affects coxsackievirus replication, persistence, and reactivation in vitro". Journal of Virology. 76 (9): 4430–4440. ISSN 0022-538X. PMID 11932410.
- ↑ Feuer, Ralph; Ruller, Chelsea M.; An, Naili; Tabor-Godwin, Jenna M.; Rhoades, Ross E.; Maciejewski, Sonia; Pagarigan, Robb R.; Cornell, Christopher T.; Crocker, Stephen J. (September 2009). "Viral persistence and chronic immunopathology in the adult central nervous system following Coxsackievirus infection during the neonatal period". Journal of Virology. 83 (18): 9356–9369. doi:10.1128/JVI.02382-07. ISSN 1098-5514. PMC 2738251. PMID 19570873.
- ↑ Lévêque, Nicolas; Renois, Fanny; Talmud, Déborah; Nguyen, Yohan; Lesaffre, François; Boulagnon, Camille; Bruneval, Patrick; Fornes, Paul; Andréoletti, Laurent (October 2012). "Quantitative genomic and antigenomic enterovirus RNA detection in explanted heart tissue samples from patients with end-stage idiopathic dilated cardiomyopathy". Journal of Clinical Microbiology. 50 (10): 3378–3380. doi:10.1128/JCM.01612-12. ISSN 1098-660X. PMC 3457437. PMID 22837323.
- ↑ Drescher, Kristen M.; Kono, Ken; Bopegamage, Shubhada; Carson, Steven D.; Tracy, Steven (November 24, 2004). "Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection". Virology. 329 (2): 381–394. doi:10.1016/j.virol.2004.06.049. ISSN 0042-6822. PMID 15518817.
- ↑ 95.0 95.1 Tracy, Steven (2014). "Human enteroviruses and type 1 diabetes" (PDF). Journal of IiME. 8 (1): 27–33.
- ↑ Smithee, S.; Tracy, S.; Chapman, N.M. (December 2015). "Mutational Disruption of cis-Acting Replication Element 2C in Coxsackievirus B3 Leads to 5'-Terminal Genomic Deletions". Journal of Virology. 89 (23): 11761–11772. doi:10.1128/JVI.01308-15. ISSN 1098-5514. PMC 4645312. PMID 26355088.
CVB3-CKO replicated to a level approximately 5 log units lower than that for wt CVB3
- ↑ 97.0 97.1 Lévêque, Nicolas; Garcia, Magali; Bouin, Alexis; Nguyen, Joseph H.C.; Tran, Genevieve P.; Andreoletti, Laurent; Semler, Bert L. (August 15, 2017). "Functional Consequences of RNA 5'-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation". Journal of Virology. 91 (16). doi:10.1128/JVI.00423-17. ISSN 1098-5514. PMC 5533909. PMID 28539455.
Taken together, our cell culture reporter transfection experiments and in vitro assays demonstrated the ability of TD RNAs harboring deletions of up to 49 nucleotides to synthesize viral proteins. CVB protein expression could, in part, be involved in DCM pathophysiology due to the expression of the viral 2A proteinase in infected cardiomyocytes. ... It should be noted that overall viral protein expression by TD replicons remained lower than that of wild-type RNAs. ... Our in vitro replication experiments did not show detectable levels of RNA synthesis for deletions larger than 21 nucleotides. ... The wild-type virus could also play the role of helper virus, allowing TD viruses to replicate
- ↑ Flynn, Claudia T.; Kimura, Taishi; Frimpong-Boateng, Kwesi; Harkins, Stephanie; Whitton, J. Lindsay (December 2017). "Immunological and pathological consequences of coxsackievirus RNA persistence in the heart". Virology. 512: 104–112. doi:10.1016/j.virol.2017.09.017. ISSN 1096-0341. PMC 5653433. PMID 28950225.
Following inoculation into mice, these variants could be identified in the heart, where they persisted but – mirroring their behavior in tissue culture – they did not trigger any detectable pathology. ... These TD viruses are non-cytopathic, and their inoculation in vivo does not initiate detectable disease. However, even if unable to kill infected cells, or to trigger disease de novo, in principle these TD viruses could exacerbate existing disease, e.g. by prolonging immunopathogenic responses to the original infection.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Maryland, US, June 2008. Timecode: 13:53". YouTube.
- ↑ Nayak, D. P.; Sivasubramanian, N. (1983). "The Structure of Influenza Virus Defective Interfering (DI) RNAs and Their Progenitor Genes". Genetics of Influenza Viruses. Vienna: Springer Vienna. pp. 255–279. doi:10.1007/978-3-7091-8706-7_8. ISBN 9783709187081.
- ↑ Dimmock, N.J. (October 1991). "The biological significance of defective interfering viruses". Reviews in Medical Virology. 1 (3): 165–176. doi:10.1002/rmv.1980010306. ISSN 1052-9276.
- ↑ Chapman, Nora (2008). "How Does a Lytic Enterovirus Persist and Cause Chronic Disease? Enterovirus Session, International Symposium on Viruses in CFS & Post-viral Fatigue, Baltimore, Maryland, US, June 2008". YouTube.
- ↑ Chapman, Nora (2010). "Invest in ME London Conference: Persistent Enteroviral Infections". Invest in ME Research.
- ↑ Tracy and Chapman (2009). "Human Enteroviruses and Chronic Infectious Disease" (PDF). Journal of IiME. 3 (1): 23–31.