Hypothalamic-pituitary-adrenal axis
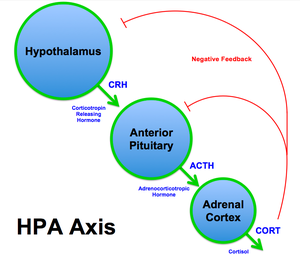
The hypothalamic–pituitary–adrenal axis (HPA axis or HTPA axis) is a complex set of direct influences and feedback interactions among three components of the neuroendocrine system: the hypothalamus, the pituitary gland, and the adrenal glands. The primary function of the HPA axis is to maintain daily metabolic homeostasis, it helps regulate many body processes, including digestion, the immune system, mood and emotions, sexuality, and energy storage and expenditure. The HPA axis is notably responsive to psychological stress and anticipated activity levels in a feed-forward manner.
Feedback loop[edit | edit source]
The negative feedback loop of the HPA axis can be described by these three components:
- The paraventricular nucleus of the hypothalamus contains neuroendocrine neurons that synthesize and secrete vasopressin and corticotropin-releasing hormone (CRH).
- These two peptides regulate the secretion of adrenocorticotropic hormone (ACTH) by the pituitary gland. ACTH in turn acts on the adrenal cortex, which produces glucocorticoid hormones (mainly cortisol in humans as well as mineralocorticoids like aldosterone) in response to stimulation by ACTH.
- Glucocorticoids in turn act back on the hypothalamus and pituitary to suppress CRH and ACTH production in a negative feedback cycle.[citation needed]
In the brain, cortisol acts on two types of receptor – mineralocorticoid receptors and glucocorticoid receptors, and these are expressed by many different types of neurons. Vasopressin can be thought of as "water conservation hormone" and is also known as "antidiuretic hormone." It is released when the body is dehydrated and has potent water-conserving effects on the kidney. It is also a potent vasoconstrictor.
Role in the body[edit | edit source]
The HPA axis has a central role in regulating many homeostatic systems in the body, including the metabolic system, cardiovascular system, immune system, reproductive system and central nervous system. The HPA axis integrates physical and psychosocial influences to anticipate metabolic demands, in order to allow an organism to adapt effectively to its environment, use resources, and optimize survival.[1]
The release of CRH from the hypothalamus is concerned with maintaining metabolic homeostasis and is primarily determined by the sleep/wake cycle (circadian rhythm), but is also moderated by psychological stress, physical activity, illness and through inhibitory feedback from elevated cortisol levels in the blood. In healthy individuals, cortisol rises rapidly after wakening, reaching a peak within 30–45 minutes. It then gradually falls over the day, rising again in late afternoon. Cortisol levels then fall in late evening, reaching a trough during the middle of the night. This corresponds to the rest-activity cycle of the organism.[1] An abnormally flattened circadian cortisol cycle has been linked with chronic fatigue syndrome.[2] However, this flattening can be explained by altered circadian rhythm and lower physical activity levels.[3]
Anatomical connections between brain areas such as the amygdala, hippocampus, prefrontal cortex and hypothalamus facilitate increased activation of the HPA axis.[4][5] Increased activation of the HPA axis can occur both reflexively in response to physical challenge and may also occur in the absence of physical challenges in an anticipatory manner.[5] Responses to physical challenges are initiated by stimuli that threaten metabolic homeostasis and can result from stimulation of ascending brain systems or circumventricular organs, which in turn project to the hypothalamic paraventricular nucleus.[5] Anticipatory responses on the other hand, are initiated either by innate responses, for example, instinctual fear, or emotional responses to the recall of prior experiences, in both cases preparing the organism to homeostatic challenges. These anticipatory responses require activation the limbic system or other systems that can anticipate potential homeostatic threat and signal to the paraventricular nucleus through indirect connections.[5]
Glucocorticoids have many important functions, including modulation of stress reactions, but in excess they can be damaging. Atrophy of the hippocampus in humans and animals exposed to severe stress is believed to be caused by prolonged exposure to high concentrations of glucocorticoids. Deficiencies of the hippocampus may reduce the memory resources available to help a body formulate appropriate reactions to stress.
Immune system[edit | edit source]
There is bi-directional communication and feedback between the HPA axis and immune system[6][7]. A number of cytokines, such as IL-1, IL-6, IL-10 and TNF-alpha can activate the HPA axis, although IL-1 is the most potent. The HPA axis in turn modulates the immune response: high levels of cortisol suppress immune and inflammatory reactions and trigger immune cells such as monocytes and neutrophils to release anti-inflammatory cytokines (e.g. IL-4, IL-10, and IL-13). [7][8][9][10] This helps to protect the organism from a potentially lethal overactivation of the immune system, and minimizes tissue damage from inflammation.[11]
Role in human disease[edit | edit source]
The HPA axis controls our body's responses to physical or emotional stress, and is being investigated as a possible cause for Myalgic encephalomyelitis.[12] Deficiencies in the HPA axis may play a role in allergies and inflammatory/ autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis.[6][7][9]
The relationship between chronic stress and its concomitant activation of the HPA axis, and dysfunction of the immune system is unclear; studies have found both immunosuppression and hyperactivation of the immune response.[10]
In ME/CFS[edit | edit source]
Notable studies[edit | edit source]
- 1996, Dissociation of body-temperature and melatonin secretion circadian rhythms in patients with chronic fatigue syndrome
- 2000, Chronic Fatigue Syndrome: A Dysfunction of the Hypothalamic-Pituitary-Adrenal Axis[13] (Abstract)
- 2018, High-fidelity discrete modeling of the HPA axis: a study of regulatory plasticity in biology[14] (Abstract)
See also[edit | edit source]
Learn more[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 Besedovsky, Hugo; Chrousos, George; Rey, Adriana Del (2008). The hypothalamus-pituitary-adrenal axis (1st ed.). Amsterdam: Academic. ISBN 9780444530400.
- ↑ MacHale SM, Cavanagh JT, Bennie J, Carroll S, Goodwin GM, Lawrie SM (November 1998). "Diurnal variation of adrenocortical activity in chronic fatigue syndrome". Neuropsychobiology. 38 (4): 213–7. doi:10.1159/000026543. PMID 9813459.
- ↑ Chung, Sooyoung; Son, Gi Hoon; Kim, Kyungjin (May 1, 2011). "Circadian rhythm of adrenal glucocorticoid: Its regulation and clinical implications". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1812 (5): 581–591. doi:10.1016/j.bbadis.2011.02.003. ISSN 0925-4439.
- ↑ Veer, Ilya M.; Oei, Nicole Y.L.; Spinhoven, Philip; van Buchem, Mark A.; Elzinga, Bernet M.; Rombouts, Serge A. R.B. (July 1, 2012). "Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex". Psychoneuroendocrinology. 37 (7): 1039–1047. doi:10.1016/j.psyneuen.2011.12.001. ISSN 0306-4530.
- ↑ 5.0 5.1 5.2 5.3 Jankord, Ryan; Herman, James P. (December 2008). "LIMBIC REGULATION OF HYPOTHALAMO-PITUITARY-ADRENOCORTICAL FUNCTION DURING ACUTE AND CHRONIC STRESS". Annals of the New York Academy of Sciences. 1148: 64–73. doi:10.1196/annals.1410.012. ISSN 0077-8923. PMC 2637449. PMID 19120092.
- ↑ 6.0 6.1 Marques-Deak, A; Cizza, G; Sternberg, E (February 2005). "Brain-immune interactions and disease susceptibility" (PDF). Molecular Psychiatry. 10: 239–250. doi:10.1038/sj.mp.4001643. Retrieved February 13, 2016.
- ↑ 7.0 7.1 7.2 Otmishi, Peyman; Gordon, Josiah; El-Oshar, Seraj; Li, Huafeng; Guardiola, Juan; Saad, Mohamed; Proctor, Mary; Yu, Jerry (2008). "Neuroimmune Interaction in Inflammatory Diseases" (PDF). Clinical Medicine: Circulatory, Respiratory, and Pulmonary Medicine. 2: 35–44. PMID 21157520. Retrieved February 14, 2016.
- ↑ Tian, Rui; Hou, Gonglin; Li, Dan; Yuan, Ti-Fei (June 2014). "A Possible Change Process of Inflammatory Cytokines in the prolonged Chronic Stress and its Ultimate Implications for Health" (PDF). The Scientific World Journal: 1–8. doi:10.1155/2014/780616. PMID 24995360. Retrieved February 13, 2016.
- ↑ 9.0 9.1 Bellavance, Marc-Andre; Rivest, Serge (March 2014). "The HPA-immune axis and the immunomodulatory actions of glucocorticoids in the brain" (PDF). Frontiers in Immunology. 5: 1–13. doi:10.3389/fimmu.2014.00136. Retrieved February 11, 2016.
- ↑ 10.0 10.1 Padgett, David; Glaser, Ronald (August 2003). "How stress influences the immune response" (PDF). Trends in Immunology. 24 (8): 444–448. doi:10.1016/S1471-4906(03)00173-X. PMID 12909458. Retrieved February 12, 2016.
- ↑ Besedovsky, Hugo; Chrousos, George; Rey, Adriana Del (2008). The hypothalamus-pituitary-adrenal axis (1st ed.). Amsterdam: Academic. ISBN 9780444530400.
- ↑ CDC (July 14, 2017). "Possible Causes | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) | CDC". Centers for Disease Control and Prevention. Retrieved October 5, 2018.
- ↑ Addington, John W. (January 1, 2000). "Chronic Fatigue Syndrome". Journal of Chronic Fatigue Syndrome. 7 (2): 63–74. doi:10.1300/J092v07n02_06. ISSN 1057-3321.
- ↑ Sedghamiz, Hooman; Morris, Matthew; Craddock, Travis J.A.; Whitley, Darrell; Broderick, Gordon (July 17, 2018). "High-fidelity discrete modeling of the HPA axis: a study of regulatory plasticity in biology". BMC Systems Biology. 12 (76). doi:10.1186/s12918-018-0599-1.

