Low dose naltrexone
Low Dose Naltrexone (LDN) refers to very small doses of the drug naltrexone hydrochloride, which at higher doses treats drug or alcohol dependence. Low dose naltrexone may reduce pain, or potentially neurological symptoms. Brand names of naltrexone include ReViva, Depade, and Vivitrol.[1] LDN use other for treating drug dependence is considered off-label. Some patients report that LDN helps reduce their symptoms of ME/CFS, Long COVID, fibromyalgia (FMS), multiple sclerosis (MS), or autoimmune disease.[2][3][4][5][6] Although its mechanism of action is unclear, some have speculated that it may act as an anti-inflammatory.[7]
Prescription forms
LDN is typically prescribed using generic naltrexone hydrochloride or branded naltrexone, divided into much smaller doses. LDN may be taken in the form of liquid solution/syrup, sublingual doses or 1.5mg capsules, or a compounding pharmacy can create smaller sized capsules or tablets. LDN in the form of naltrexone cream, subcutaneous injections, IV naltrexone and eye drops (for dry eyes) are also available.[8]
VLDN and ULDN
Very Low Dose Naltrexone (VLDN) and Ultra-Low Dose Naltrexone (ULDN) have recently been used in limited trials, both VLDN and ULDN involve doses of naltrexone significantly below 1mg.[9]
Very Low Dose Naltrexone is increasingly being used under 1mg for people who cannot titrate from 0.5mg to find their individual optimal dose.
Evidence
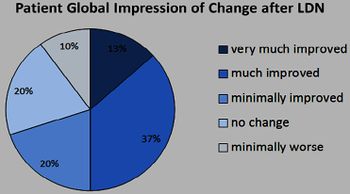
Jarred Younger published a small study that concluded "...low-dose naltrexone may be an effective, highly tolerable, and inexpensive treatment for fibromyalgia".[10][11]
A second study concluded that "specific and clinically beneficial impact on fibromyalgia pain".[12][13]
A 2014 review by Stanford researchers suggests that "LDN may operate as a novel anti-inflammatory agent in the central nervous system, via action on microglial cells. These effects may be unique to low dosages of naltrexone and appear to be entirely independent from naltrexone's better-known activity on opioid receptors. As a daily oral therapy, LDN is inexpensive and well-tolerated."[7]
The FDA approved naltrexone HCL in 1984 to treat opioid addiction. Low-dose naltrexone is typically given at about 1/10th the typical dose of naltrexone. By blocking opioid receptors, naltrexone can increase pain, but at very low doses naltrexone has both pain-reducing (analgesic) and anti-inflammatory properties.
In 2012 Solve ME/CFS Initiative contracted Biovista to use drug models to identify existing drugs that may be worth investigating for treatment. The results suggested Naltrexone was worth considering.[14]
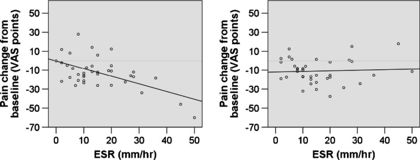
Jarred Younger's research suggests that people with an Erythrocyte Sedimentation Rate (ESR) over 40 millimeters an hour, tend to be strong responders to LDN, and that there may be other predictive factors for success.[15]
News articles
- 2019, In Tiny Doses, An Addiction Medication Moonlights As A Treatment For Chronic Pain - NPR, All Things Considered
- September 9, 2021https://www.empr.com/home/news/drugs-in-the-pipeline/low-dose-naltrexone-designated-orphan-drug-for-complex-regional-pain-syndrome/
Clinical use
Naltrexone is a prescription drug in many countries including the United States.[16]Compounding chemists or compounding pharmacists can mix naltrexone with a powder filler or dilute in into a liquid to create the lower dose.
Fast-release fillers only
The LDN Research Trust advises that: "Pharmacies should be instructed NOT to provide LDN in an "SR" or slow-release or timed-release form. Unless the low dose of naltrexone is in an unaltered form, which permits it to reach a prompt "spike" in the blood stream, its therapeutic effects may be inhibited."[17] and states that calcium carbonate filler should NOT be used because they reduce absorption, instead Avicel, lactose, or sucrose fillers as alternative fast-release fillers."[17]
Do not take with
In general, Low Dose Naltrexone (LDN) should not be taken concurrently with opioid-containing drugs (opioid receptors in brain are blocked by LDN), alcohol, immunosuppressive drugs, or immunomodulator drugs.[citation needed] LDN blocks the effect of opioid drugs.[18] Some opioid drugs are codeine, oxycodone, vicodin, hydrocodone, fentanyl and morphine.
Some people include tramadol in the list of opioid containing drugs, but tramadol works differently. Per Skip Lenz, a pharmacist and LDN expert -
From the Q&A section of the first LDN Book:
"It should be well known that LDN cannot be taken with opiate pain medications such as oxycontin or morphine. However, I have long recommended that patients who must take pain medications consider tramadol, which is not an opiate, but works on similar receptors. The only problem can be with the amount of tramadol you can take over a period of a day. Large amounts, that is, over 300 mg per day, have been reported to be problematic, but to my knowledge, lower doses (50 mg taken two or three times a day) have not presented any problems for patients while are on LDN."
This situation would only apply to people well established on LDN, as would using opioids with LDN at the opposite ends of the day.
Pharmacies
Australia
Compounding Pharmacies are able to fill these prescriptions, and post if needed.
UK
LDN suppliers in the UK include Dickson Chemist in Glasgow, Roseway Labs, and Specialist Pharmacy (The London Specialist Pharmacy Ltd). All these are compounding pharmacies and require a prescription, they are usually able to put patients in contact with private doctors who will consider writing a prescription, and can post medications to you.[19] Compounding pharmacies are regulated by the UK's General Pharmaceutical Council.[19]
United States
Neighborhood Compounding Pharmacies are able to fill these prescriptions and mail if needed. Your prescribing doctor can help you locate a compounding pharmacy in your area/state or you can look online.[20]
Other countries
Roseway Labs supplies LDN in the EU. The LDN Research Trust lists pharmacies in multiple countries.[21]
Clinical trials
A large number of clinical trials have been completed for LDN recently, including ones that have looked at the effect of LDN on symptoms of myalgic encephalomyelitis/chronic fatigue syndrome. Research has also been carried out on patients with MS, Chronic Regional Pain Syndrome, FMS, Irritable Bowel Syndrome (IBS), Ulcerative Colitis, Skin Disorders and a range of other illnesses.[22]
When, How To Take
Dr Whitaker states that the ideal dose is different for each person. Some doctors recommend starting at 1mg.[23] Common dosages are 1.5mg, 3mg, 4.5mg.[24][25][18] When beginning use of LDN, the drug must be stepped up over 6-8+ weeks as it may keep you awake; discuss how best to do this with your doctor and pharmacist.[25][26][18]
LDN is usually taken at bedtime. Some people take LDN in the morning to minimize sleep disturbance, insomnia, and vivid dreams.[18]
Talks and webinars
Notable studies
- 2009, Fibromyalgia Symptoms Are Reduced by Low-Dose Naltrexone: A Pilot Study[30] - (Full text)
- 2013, Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels[12] - (Full text)
- 2014, The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain[7] - (Full text)
- 2018, Double-blinded placebo-controlled cross-over pilot trial of naltrexone to treat Gulf War Illness[31] - (Abstract)
- 2018, Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization[32] - (Full text)
- 2019, Low-dose naltrexone in the treatment of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)[33] - (Abstract)
- 2020, Low-dose naltrexone as a treatment for chronic fatigue syndrome[34] - (Full text)
- 2021, Potential Therapeutic Benefit of Low Dose Naltrexone in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Role of Transient Receptor Potential Melastatin 3 Ion Channels in Pathophysiology and Treatment[35] - (Full text)
See also
Learn more
- Low-dose Naltrexone (LDN) Doctor's Fact Sheet 2014 - LDN Research Trust
- LDN Research Trust[36]
- Low Dose Naltrexone and chronic pain[18]
- Wikipedia - Naltrexone
- LDN Now
- LDN Science - MedInsight Research Institute
- 2015, The Use of Naltrexone in Low Doses Beyond the Approved Indication[37]
- Top 15 Scientific Health Benefits of Low Dose Naltrexone (2016)
- Low Dose Naltrexone Resource Center for Fibromylgia and ME/CFS (2019)
- 2016, Low-Dose Naltrexone as Adjunctive Pharmacotherapy for Fibromyalgia
- The LDN Book - Lisa Elsegood (2016)
- 2016, Low Dose Naltrexone Drug Combination Proposed for Chronic Fatigue Syndrome - Health Rising, by Cort Johnson (2016)
- Low dose naltrexone: side effects and efficacy in gastrointestinal disorders by CFS Remission (2016)
- Opioid blocking and alcohol - FAQ About Naltrexone Treatment for Alcoholism - 10.
- Low Dose Naltrexone for Fibromyalgia (2018)
- Low Dose Naltrexone Therapy for Crohn's Disease (2018)
- LDN LOW DOSE NALTREXONE FOR ME/CFS MYALGIC ENCEPHALOMYELITIS & FIBROMYALGIA
References
- ↑ "Naltrexone - brand name list from Drugs.com". Drugs.com. Retrieved January 17, 2022.
- ↑ Bolton, Monica Jane; Chapman, Bryan Paul; Van Marwijk, Harm (January 6, 2020). "Low-dose naltrexone as a treatment for chronic fatigue syndrome". BMJ case reports. 13 (1): e232502. doi:10.1136/bcr-2019-232502. ISSN 1757-790X. PMC 6954765. PMID 31911410.
- ↑ Bonilla, Hector; Tian, Lu; Marconi, Vincent C.; Shafer, Robert; McComsey, Grace A.; Miglis, Mitchel; Yang, Philip; Bonilla, Andres; Eggert, Lauren; Geng, Linda N. (November 1, 2023). "Low-dose naltrexone use for the management of post-acute sequelae of COVID-19". International Immunopharmacology. 124: 110966. doi:10.1016/j.intimp.2023.110966. ISSN 1567-5769. PMC 11028858. PMID 37804660.
- ↑ Toljan, Karlo; Vrooman, Bruce (September 21, 2018). "Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization". Medical Sciences. 6 (4): 82. doi:10.3390/medsci6040082. ISSN 2076-3271. PMC 6313374. PMID 30248938.
- ↑ Driver, C. Noelle; D’Souza, Ryan S. (April 3, 2023). "Efficacy of Low-Dose Naltrexone and Predictors of Treatment Success or Discontinuation in Fibromyalgia and Other Chronic Pain Conditions: A Fourteen-Year, Enterprise-Wide Retrospective Analysis". Biomedicines. 11 (4): 1087. doi:10.3390/biomedicines11041087. ISSN 2227-9059. PMC 10135963. PMID 37189705.
- ↑ Brook, Jill. "Results of the LDN Side Effects Survey" (PDF). LDN Research Trush. Retrieved June 7, 2024.
- ↑ 7.0 7.1 7.2 7.3 Younger, Jarred; Parkitny, Luke; McLain, David (February 15, 2014). "The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain". Clin Rheumatol. 33 (4): 451–459. doi:10.1007/s10067-014-2517-2. PMID 24526250.
- ↑ "Types of LDN". LDN Research Trust. Retrieved January 17, 2022.
- ↑ Toljan, Karlo; Vrooman, Bruce (September 21, 2018). "Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization". Medical Sciences. 6 (4). doi:10.3390/medsci6040082. ISSN 2076-3271. PMID 30248938.
- ↑ Younger, Jarred; Mackey, Sean (April 22, 2009). "Fibromyalgia Symptoms Are Reduced by Low-Dose Naltrexone: A Pilot Study". Pain Med. 10 (4): 663–672. doi:10.1111/j.1526-4637.2009.00613.x. PMID 2891387.
- ↑ Mackey, Sean (May 1, 2009). "An Update on Fibromyalgia". Research Channel (USA).
- ↑ 12.0 12.1 Younger, Jarred; Noor, Noorulain; McCue, Rebecca; Mackey, Sean (January 28, 2013). "Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels". Arthritis Rheum. 65 (2): 529–38. doi:10.1002/art.37734. PMID 23359310.
- ↑ "Low Dose Naltrexone for Fibromyalgia". Systems Neuroscience and Pain Lab | Stanford Medicine. Retrieved October 4, 2018.
- ↑ Solve ME/CFS Initiative. "Biovista work released".
- ↑ Younger, Jarred; Cohen, Joseph M (March 29, 2016). "Dr. Jarred Younger: Cutting Edge Research on CFS, Neuroinflammation, Pain, and Fatigue" (video interview with transcript). Self Hacked Blog.
- ↑ Feeney, G. F. X.; Connor, J.P.; Young, R. McD; Tucker, J.; Czajkowski, F. (2001). "Adherence with naltrexone prescription advice in hospital outpatient alcohol rehabilitation programme". Journal of Clinical Pharmacy and Therapeutics. 26 (1): 73–79. doi:10.1111/j.1365-2710.2001.00326.x. ISSN 1365-2710.
- ↑ 17.0 17.1 "Low-dose Naltrexone (LDN) Fact Sheet 2014" (PDF). LDN Research Trust. 2014.
- ↑ 18.0 18.1 18.2 18.3 18.4 Chopra, Pradeep. "Low Dose Naltrexone and chronic pain". LDN Research Trust. Retrieved January 31, 2019.
- ↑ 19.0 19.1 "Compounding processes regulations/". Roseway Labs.
- ↑ Finding a Compounding Pharmacy - WIKI How
- ↑ "LDN Pharmacists". LDN Research Trust.
- ↑ "Clinical Trials". LDN Research Trust. Retrieved January 21, 2019.
- ↑ LDN Now. "LDN Dosing". Retrieved February 1, 2018.
- ↑ Myers, Amy (May 2, 2017). "Low-Dose Naltrexone for Autoimmunity?". Amy Myers MD. Retrieved February 2, 2019.
- ↑ 25.0 25.1 Dr Whitaker. "What is Low-Dose Naltrexone?". drwhitaker.com. Retrieved January 21, 2019.
- ↑ "Low Dose Naltrexone (LDN) – Collier Drug Store". collierdrug.com. What dose of Low Dose Naltrexone (LDN) is best?. Retrieved February 2, 2019.
- ↑ "LDNscience® Presents - How LDN (Low Dose Naltrexone) Works". YouTube. LDNscience. December 20, 2012.
- ↑ Carnahan, Jill; Vasquez, Alex (November 30, 2015). "Functional Medicine & LDN (low-dose naltrexone) with Drs Carnahan and Vasquez". YouTube. Alex Vasquez.
- ↑ "1:05 / 7:05 Is Low Dose Naltrexone (LDN) for you?". YouTube. October 11, 2015.
- ↑ Younger, Jarred; Mackey, Sean (May 2009). "Fibromyalgia Symptoms Are Reduced by Low-Dose Naltrexone: A Pilot Study". Pain Medicine. 10 (4): 663–672. doi:10.1111/j.1526-4637.2009.00613.x. ISSN 1526-2375. PMC 2891387. PMID 19453963.
- ↑ Brewer, Kori L.; Mainhart, Allison; Meggs, William J. (2018). "Double-blinded placebo-controlled cross-over pilot trial of naltrexone to treat Gulf War Illness". Fatigue: Biomedicine, Health & Behavior. 6 (3): 132–140. doi:10.1080/21641846.2018.1477034.
- ↑ Toljan, Karlo; Vrooman, Bruce (September 21, 2018). "Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization". Medical Sciences. 6 (4): 82. doi:10.3390/medsci6040082. ISSN 2076-3271. PMC 6313374. PMID 30248938.
- ↑ Polo, Olli; Pesonen, Pia; Tuominen, Essi (November 19, 2019). "Low-dose naltrexone in the treatment of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)". Fatigue: Biomedicine, Health & Behavior. 7 (4): 207–217. doi:10.1080/21641846.2019.1692770. ISSN 2164-1846.
- ↑ Bolton, Monica Jane; Chapman, Bryan Paul; Van Marwijk, Harm (January 2020). "Low-dose naltrexone as a treatment for chronic fatigue syndrome". BMJ Case Reports. 13 (1): e232502. doi:10.1136/bcr-2019-232502. ISSN 1757-790X. PMC 6954765. PMID 31911410.
- ↑ Cabanas, Helene; Muraki, Katsuhiko; Eaton-Fitch, Natalie; Staines, Donald Ross; Marshall-Gradisnik, Sonya (2021). "Potential Therapeutic Benefit of Low Dose Naltrexone in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Role of Transient Receptor Potential Melastatin 3 Ion Channels in Pathophysiology and Treatment". Frontiers in Immunology. 12: 687806. doi:10.3389/fimmu.2021.687806. ISSN 1664-3224. PMC 8313851. PMID 34326841.
- ↑ "Low Dose Naltrexone |". LDN Research Trust. Retrieved February 2, 2019.
- ↑ Ringerike, Tove; Pike, Eva; Nevjar, Janicke; Klemp, Marianne (2015), The Use of Naltrexone in Low Doses Beyond the Approved Indication, NIPH

