Positron emission tomography
Positron emission tomography (PET) is a method of biomedical imaging that uses nuclear functional imaging techniques to observe metabolic processes in the body. In clinical settings, it is predominantly used in oncology for tumor metastasis imaging, neurology, and cardiology.[1]
Basics of PET[edit | edit source]
Radiotracers[edit | edit source]
PET uses radiotracers (also called radioactive tracers or radioligands), which are biological compounds that have been altered to include a radioactive atom (called a positron-emitting radioisotope). Radiotracers are biologically active, meaning they are designed to interact with your body, often by binding to a particular chemical or receptor.[2]
The type of radiotracer used depends on what metabolic process the doctor wants to look at. For example, fludeoxyglucose (FDG) is a radiotracer that mimics glucose. Places where FDG congregate tend to have increased glucose uptake, a sign that the region has increased metabolic activity. This can be helpful for detecting tumors, which need more energy to spread through the body.[3]
The process[edit | edit source]
The radiotracer is injected into the subject's body, and the subject enters the PET machine. Over time, the radiotracer will break down, emitting gamma rays. The machine picks up these rays and translates them into a 3-dimensional image showing where the radiotracer is concentrated in the body.[2] The resulting image can be thought of as a heat map, showing areas of high concentration as more brightly lit.
Risks[edit | edit source]
The risks for the amount of radiotracer injected into the body is small enough that there is usually no need to take precautions against radioactive exposure. If you are pregnant or breastfeeding, you should notify the doctor/researcher to protect against injury to the fetus or contaminating breastmilk.[4]
Clinical reasons for getting a PET scan:[edit | edit source]
PET is often used to evaluate the function of organs such as the heart and brain. Common uses include[4]:
- To measure perfusion of the heart muscle
- To diagnose neurological conditions such as Alzheimer’s, Huntington’s, Parkinson’s, epilepsy, and stroke
- To detect the spread of cancer or evaluate cancer treatment efficacy
- To locate the specific site for surgery prior to the surgical procedure
- To evaluate the brain after trauma
PET research in ME/CFS[edit | edit source]
PET research in ME has focused on measures of neuroinflammation. This has been done using a radiotracer that binds to the translocator protein (TSPO). Microglia, the resident macrophages of the brain, produce TSPO when they activate.[5] Microglial activation is a commonly used measure of neuroinflammation.
Nakatomi et al. 2014[edit | edit source]
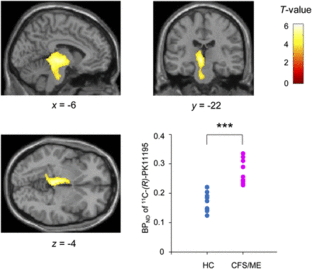
Nakatomi et al. (2014) was the first case-control study using PET to measure neuroinflammation through TSPO expression in ME. They used PK11195, a first-generation TSPO-binding radioligand. They found increased PK11195 in cingulate cortex, hippocampus, amygdala, thalamus, midbrain, and pons. PK11195 concentrations in certain regions had positive correlations with cognitive impairment scores (related to brain fog), pain scores, and depression scores. The study concludes that neuroinflammation is associated with neuropsychological symptoms in ME patients.[6]
PET research in long COVID[edit | edit source]
Sollini et al. 2021[edit | edit source]
Sollini et al. (2021) compared 13 adult long COVID patients to a group of 26 melanoma patients with a negative PET/CT. COVID patients were matched for sex/age.
- In 4/13 long COVID patients, CT images showed lung abnormalities presenting mild [18F]FDG uptake.
- Long COVID patients also had brain hypometabolism in the right parahippocampal gyrus and thalamus (uncorrected p ≤ 0.001).
This study concluded that [18F}FDG PET/CT can be a tool to analyze the multi-organ nature of long COVID.[7]
Guedj et al. 2021.[edit | edit source]
Guedj et al. (2021) completed PET scans for 35 long COVID patients which utilized a whole-brain voxel-based analysis. They compared these patients to a local database of 44 health subjects which were controlled by age and sex. Long COVID patients exhibited bilateral hypometabolism compared to health patients. Study supports that PET scans may be valuable for long COVID patients in order to perform a whole-brain voxel-based analysis. Additionally, prevalence of hypometabolism was statistically greater for COVID patients compared to healthy patients.[8]
See also[edit | edit source]
References[edit | edit source]
- ↑ Bar-Shalom, Rachel; Valdivia, Ana Y.; Blaufox, M. Donald (July 1, 2000). "PET imaging in oncology". Seminars in Nuclear Medicine. Entering a New Millennium. 30 (3): 150–185. doi:10.1053/snuc.2000.7439. ISSN 0001-2998.
- ↑ 2.0 2.1 Alauddin, Mian M (December 15, 2011). "Positron emission tomography (PET) imaging with 18F-based radiotracers". American Journal of Nuclear Medicine and Molecular Imaging. 2 (1): 55–76. ISSN 2160-8407. PMC 3478111. PMID 23133802.
- ↑ Annibaldi, A.; Widmann, C. (July 2010). "Glucose metabolism in cancer cells". Current Opinion in Clinical Nutrition and Metabolic Care. pp. 466–470. doi:10.1097/MCO.0b013e32833a5577.
- ↑ 4.0 4.1 "How Does a PET Scan Work?". Johns Hopkins Medicine. Retrieved February 27, 2019.
- ↑ Lara Mejia, Paula S.; Brumfield, Sydney A.; VanElzakker, Michael B. (2019). "Neuroinflammation and Cytokines in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Critical Review of Research Methods". Frontiers in Neurology. 9. doi:10.3389/fneur.2018.01033. ISSN 1664-2295.
- ↑ 6.0 6.1 Nakatomi, Yasuhito (June 1, 2014). Kazuhiro Takahashi; Yosky Kataoka; Susuma Shiomi; Kouzi Yamaguti, Masaaki Inaba; Hirohiko Kuratsune; Yasuyoshi Watanabe. "Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An 11C-(R)-PK11195 PET Study". The Journal of Nuclear Medicine. 555 (6): 945–950 – via SNM Journals.
- ↑ Sollini, Martina; Morbelli, Silvia; Ciccarelli, Michele; Cecconi, Maurizio; Aghemo, Alessio; Morelli, Paola; Chiola, Silvia; Gelardi, Fabrizia; Chiti, Arturo (March 7, 2021). "Long COVID hallmarks on [18F]FDG-PET/CT: a case-control study". European Journal of Nuclear Medicine and Molecular Imaging. doi:10.1007/s00259-021-05294-3. ISSN 1619-7070. PMC 7937050. PMID 33677642.
- ↑ Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P. (January 26, 2021). "18F-FDG brain PET hypometabolism in patients with long COVID". European Journal of Nuclear Medicine and Molecular Imaging. doi:10.1007/s00259-021-05215-4. ISSN 1619-7070. PMC 7837643. PMID 33501506.

