Stem cell therapy
From MEpedia, a crowd-sourced encyclopedia of ME and CFS science and history
This article is a stub. |
Stem cell therapy, in particular the use of mesenchymal stromal cells (MSCs) is a potential treatment that has been suggested for ME/CFS.[1]
Theory[edit | edit source]
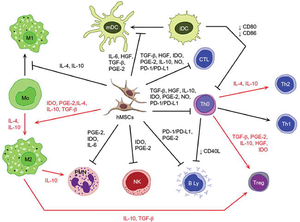
iDC, immature dendritic cell; IL, interleukin;
HGF, hepatocyte growth factor;
TGF-β, transforming growth factor-β;
PGE-2, prostaglandin E2; IDO, indoleamine-2,3-dioxygenase;
NO, nitric oxide; PD-L1, programmed death-ligand 1;
hMSC, human mesenchymal stem cell;
Treg, T regulatory; Th, T helper cell
CTL, cytotoxic T cell; mDC, mature dendritic cell
PD-1, programmed cell death protein ;1
PMN, polymorphonuclear leukocyte;
NK, Natural killer cell
HGF, hepatocyte growth factor;
TGF-β, transforming growth factor-β;
PGE-2, prostaglandin E2; IDO, indoleamine-2,3-dioxygenase;
NO, nitric oxide; PD-L1, programmed death-ligand 1;
hMSC, human mesenchymal stem cell;
Treg, T regulatory; Th, T helper cell
CTL, cytotoxic T cell; mDC, mature dendritic cell
PD-1, programmed cell death protein ;1
PMN, polymorphonuclear leukocyte;
NK, Natural killer cell
Evidence[edit | edit source]
Clinical trials of stem cell therapies have not yet been published.[1]
Clinicians[edit | edit source]
Risks and safety[edit | edit source]
Costs and availability[edit | edit source]
See also[edit | edit source]
- Immune system
- Metabolic trap
- Indoleamine-2,3-dioxygenase
- Interleukin
- transforming growth factor-β;
- Nitric oxide
- T regulatory
- T helper cell
- Cytotoxic T cell
- Natural killer cell
Learn more[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 Moreno-Manzano, Victoria; García, Elisa Oltra (November 5, 2018). "Culturing Adult Stem Cells for Cell-Based Therapeutics: Neuroimmune Applications". Cell Culture. IntechOpen. doi:10.5772/intechopen.80714.

