Pregabalin
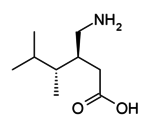
Pregabalin, marketed under the brand name Lyrica among others, is a medication used to treat epilepsy, neuropathic pain, fibromyalgia, generalized anxiety disorder, diabetic nerve pain, pain after shingles, spinal cord injury nerve pain, and partial onset seizures in adults (an add-on treatment) with epilepsy. It is very similar to gabapentin, both chemically and pharmacologically.
Lyrica® is manufactured by the US pharmaceutical company, Pfizer, Inc.[1]
Side Effects[edit | edit source]
This information contains side effect information about pregabalin, Lyrica's active ingredient. Some of the dosage forms listed on this page may not apply to the brand name Lyrica.
Always review a drug company's prescription drug's side effects and possible drug interactions with your doctor and pharmacist.
Pregnant women must always consult with their doctor and pharmacist when taking any prescription drug, over-the-counter drug, supplement, or herbal remedy for side effects, drug interactions, and possible miscarriage, injury, birth defect, addiction, or death to the embryo, fetus, or baby. Breastfeeding women should always talk to their doctor and pharmacist for information about breast milk being laced with the drug she has taken causing side effects, injury, addiction, or death, and drug interactions to the baby consuming her breast milk.
Check with your doctor and pharmacist before using any prescription or over-the-counter drug, supplement, or herbal remedy being administered to a child. Check with your doctor and pharmacist for children's side effects or drug interactions that may not be listed.
More commonly reported side effects[edit | edit source]
- Accidental injury
- bloating or swelling of the face, arms, hands, lower legs, or feet
- blurred vision
- burning, tingling, numbness or pain in the hands, arms, feet, or legs
- change in walking and balance
- clumsiness
- confusion
- dementia
- difficulty having a bowel movement
- difficulty with speaking
- double vision
- dry mouth
- false beliefs that cannot be changed by facts
- fever
- headache
- hoarseness
- increased appetite
- lack of coordination
- loss of memory
- lower back or side pain
- painful or difficult urination
- problems with memory
- rapid weight gain
- sensation of pins and needles
- shakiness
- sleepiness
- stabbing pain
- trembling, or other problems with muscle control or coordination
- unsteady walk
- unusual drowsiness
- unusual weight gain or loss[2]
Less commonly reported ones are:[edit | edit source]
- Anxiety
- bloated or feeling of fullness
- chest pain
- cold sweats
- cool, pale skin
- cough producing mucus
- decrease or change in vision
- depression
- excess air or gas in the stomach or intestines
- eye disorder
- false or unusual sense of well-being
- general feeling of discomfort or illness
- increased hunger
- loss of appetite
- loss of bladder control
- loss of consciousness
- loss of strength or energy
- muscle aches, twitching or jerking, or weakness
- nausea
- nervousness
- nightmares
- noisy breathing
- pain
- passing gas
- rhythmic movement of the muscles
- runny nose
- seizures
- shivering
- slurred speech
- sweating
- trouble sleeping
- twitching
- uncontrolled eye movements
- vomiting[2]
Other less commonly reported ones are:[edit | edit source]
- Difficult or labored breathing
- tightness in the chest[2]
Rarely reported ones are:[edit | edit source]
- Blistering, peeling, or loosening of the skin
- chills
- cough
- diarrhea
- difficulty with swallowing
- dizziness
- fast heartbeat
- hives, itching, skin rash
- joint or muscle pain
- puffiness or swelling of the eyelids or around the eyes, face, lips, or tongue
- red skin lesions, often with a purple center
- red, irritated eyes
- sore throat
- sores, ulcers, or white spots in the mouth or on the lips
- unusual tiredness or weakness[2]
Generic legal battle[edit | edit source]
- 2012, Pfizer blocked multiple drug manufacturers from selling pregabalin, a generic version of Pfizer's drug Lyrica.[3]
- July 2012, the U.S. Food and Drug Administration (FDA) approved a generic version of Lyrica. Pregabalin, the active ingredient, is the generic name.[4] Two weeks after being approved by the FDA, marketing was blocked by Pfizer.[3]
- 2014, Pfizer Inc., the maker of Lyrica, successfully blocked the marketing of Pregabalin generics until December of 2018 arguing that the generics should not be marketed and made available until their patent ran out.[5] Warner-Lamber, (a subsidiary of Pfizer), "still holds a “second medical use” patent for the use of pregabalin in the treatment of peripheral and central neuropathic pain, which expires in July 2017. A second medical use patent is one that relates to a new medical use for a known compound."[6]
- Nov 2018, Pfizer Receives Six Months Pediatric Exclusivity For Lyrica® (Pregabalin)[7]
Pfizer Inc. (NYSE: PFE) today announced that the U.S. Food and Drug Administration (FDA) has granted pediatric exclusivity for LYRICA®. This grant extends the period of U.S. market exclusivity for LYRICA by an additional six months, to June 30, 2019.[7]
- July 22, 1019, FDA approves first generics of Lyrica[8]
On July 19, the U.S. Food and Drug Administration approved multiple applications for first generics of Lyrica (pregabalin) for the management of neuropathic pain associated with diabetic peripheral neuropathy, for the management of postherpetic neuralgia, as an adjunctive therapy for the treatment of partial onset seizures in patients 17 years of age and older, for the management of fibromyalgia, and for the management of neuropathic pain associated with spinal cord injury.[8]
Lyrica patient assistance[edit | edit source]
These assistance programs come with various insurance coverage limitations and income thresholds.
- Co-pay Card
- Patient Assistance Program Check eligibility on Pfizer Rx Pathways
See also[edit | edit source]
- Fibromyalgia
- Neurontin (Gabapentin)
- Baclofen
Learn more[edit | edit source]
- Alan Light - Segment 3: Update on Dr. Light's Recent CFS and FM Research[9]
- Frequently Asked Questions by Lyrica® FAQ's for each condition Lyrica is prescribed for.
- Lyrica and Cymbalta Recommended for Diabetic Neuropathy[10] (Diabetic nerve pain.)
- Lyrica for Fibromyalgia[11]
- How does Lyrica treat nerve pain from shingles?[12]
- FDA Approves Lyrica For The Management Of Neuropathic Pain Associated With Spinal Cord Injury Based On Priority Review[13]
- FDA Approves Pregabalin as Epilepsy Add-On Treatment for Partial Onset Seizures[14]
References[edit | edit source]
- ↑ "LYRICA® and LYRICA®CR (pregabalin) and (pregabalin extended release tablets) | Pfizer: One of the world's premier biopharmaceutical companies". pfizer.com. Retrieved August 12, 2018.
- ↑ 2.0 2.1 2.2 2.3 "Lyrica Side Effects in Detail - Drugs.com". Drugs.com. Retrieved August 12, 2018.
- ↑ 3.0 3.1 "Court upholds Pfizer's Lyrica patent protection". Reuters. July 19, 2012. Retrieved March 9, 2021.
- ↑ "U.S. OKs Lupin generic of Pfizer nerve pain drug". Reuters. July 5, 2012. Retrieved March 9, 2021.
- ↑ Decker, Susan (February 6, 2014). "Pfizer Wins Ruling to Block Generic Lyrica Until 2018". Bloomberg Business.
- ↑ Wise, Jacqui (March 30, 2015). "Doctors are warned not to prescribe generic pregabalin for pain control". BMJ. 350: h1724. doi:10.1136/bmj.h1724. ISSN 1756-1833. PMID 25825288.
- ↑ 7.0 7.1 "Pfizer Receives Six Months Pediatric Exclusivity for LYRICA® (pregabalin)". investors.pfizer.com. November 27, 2018. Retrieved December 27, 2018.
- ↑ 8.0 8.1 Commissioner, Office of the (September 11, 2019). "FDA approves first generics of Lyrica". FDA. Retrieved October 11, 2019.
- ↑ "Alan Light - Segment 3: Update on Dr. Light's Recent CFS and FM Research". YouTube. Richard Podell. February 22, 2016.
- ↑ Anson, Pat (January 31, 2017). "Lyrica and Cymbalta Advised for Diabetic Neuropathy". Pain News Network. Retrieved August 12, 2018.
- ↑ "Lyrica for Fibromyalgia Treatment". WebMD. Retrieved August 12, 2018.
- ↑ Wiegman, Stacy. "How does Lyrica treat nerve pain from shingles? | Medications to Treat Fibromyalgia". Sharecare. Retrieved August 12, 2018.
- ↑ "FDA Approves Lyrica For The Management Of Neuropathic Pain Associated With Spinal Cord Injury Based On Priority Review | Pfizer Pharmaceutical News and Media | Pfizer: the world's largest research-based pharmaceutical company". press.pfizer.com. Retrieved August 12, 2018.
- ↑ "FDA Approves Pfizer's Lyrica as Epilepsy Add-On Treatment for Partial Onset Seizures". Business Wire. January 13, 2005. Retrieved August 12, 2018.

