Ampligen Exercise Tolerance - graph 1
From MEpedia, a crowd-sourced encyclopedia of ME and CFS science and history
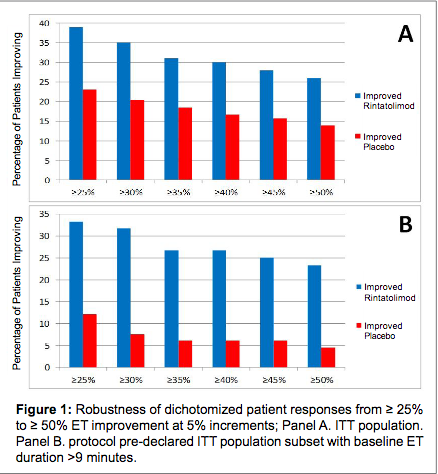
Graph from study, Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME): Characteristics of Responders to Rintatolimod.[1]
Key to graph
- "Rintatolimod" - chemical name for Ampligen
- "ET" - exercise tolerance measured by seconds on a treadmill using a modified Bruce exercise test (i.e., a cardiac diagnostic test which starts at a lower workload than the standard test and is typically used for elderly or sedentary patients)[2]
- "ITT" - intent-to-treat population which is a statistical concept term used in studies to denote that the analysis includes every subject who was randomly placed in a treatment assignment group, and ignores noncompliance, protocol deviations, withdrawal, and anything that happens after randomization. It is considered a more accurate way to analyze data since noncompliance and protocol deviations would occur during clinical use.[3]
Interpretation of graph
- Panel A - total study population retested at 40 weeks
- Panel B - subset of study population retested at 40 weeks who could complete >9 minutes using a modified Bruce exercise test prior to receiving rintatolimod
See also
References
- ↑ Strayer, David R; Stouch, Bruce C; Stevens, Staci R.; Bateman, Lucinda; Lapp, Charles W; Peterson, Daniel L; Carter, William A; Mitchell, William M (August 8, 2015), "Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME): Characteristics of Responders to Rintatolimod" (PDF), Journal of Drug Research and Development, 1 (1), doi:10.16966/2470-1009.103
- ↑ https://en.wikipedia.org/wiki/Bruce_protocol
- ↑ Gupta, SK (2011), "Intention-to-treat concept: A review.", Perspectives in Clinical Research, 2 (3): 109-112, doi:10.4103/2229-3485.83221

