Evidence of widespread metabolite abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy (2019) Mueller, et al: Difference between revisions
Notjusttired (talk | contribs) (→Authors: convert web ref to journal citation, add journal name) |
Notjusttired (talk | contribs) m (→Abstract and Conclusion: add links, remove links to pages we aren't likely to create - use glossary instead) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
== Authors == | == Authors == | ||
[[Christine Mueller|Christina Mueller]] (MS), [[Joanne Lin]], [[Sulaiman Sheriff]], [[Andrew Maudsley]], under the direction of Dr. [[Jarred Younger]] of the Neuroinflammation, Pain and Fatigue Laboratory at University of Alabama at Birmingham and with University of Miami Miller School of Medicine radiology experts.<ref name=" | [[Christine Mueller|Christina Mueller]] (MS), [[Joanne Lin]], [[Sulaiman Sheriff]], [[Andrew Maudsley]], under the direction of Dr. [[Jarred Younger]] of the Neuroinflammation, Pain and Fatigue Laboratory at University of Alabama at Birmingham and with University of Miami Miller School of Medicine radiology experts.<ref name="Mueller2019">{{Cite journal|url=https://link.springer.com/epdf/10.1007/s11682-018-0029-4|title=Evidence of widespread metabolite abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy|last=Mueller|first=Christina|authorlink=Christina Mueller|last2=Lin|first2=Joanne C|authorlink2=Joanne Lin|date=2019|doi=10.1007/s11682-018-0029-4|archive-url=|archive-date=|dead-url=|access-date=2019-01-17|authorlink3=Sulaiman Sheriff|authorlink4=Andrew Maudsley|authorlink5=Jarred Younger|last3=Sheriff|last4=Maudsley|last5=Younger|first3=Sulaiman|first4=Andrew A|first5=Jarred W|volume=|issue=|pages=|page=|journal=Brain Imaging and Behavior}}</ref><ref name="RamsayAward20190117">{{Cite web|url=http://go.solvecfs.org/webmail/192652/97033877/74d435097e87231f24cffe4d9de93da8e3f71c0e077a67417eba10cbd269ccb1|title=Brain Imaging and Behavior publication from Dr. Jarred Younger’s SMCI Ramsay pilot study supports involvement of neuroinflammation in ME/CFS|website=go.solvecfs.org|access-date=2019-01-17|date=|last=|first=|authorlink=|last2=|first2=|authorlink2=|archive-url=|archive-date=|dead-url=}}</ref> | ||
== Funding == | == Funding == | ||
This study was funded through [[Solve ME/CFS Initiative]] | This study was funded through [[Solve ME/CFS Initiative]]s' Ramsay Award Program<ref name="RamsayAward20190117" /><ref name="RamsayAward">{{Cite web|url=https://solvecfs.org/ramsay-award-program/|title=Ramsay Award Program|website=Solve ME/CFS Initiative|language=en-US|access-date=2019-01-17}}</ref> and the [[National Institutes of Health]] (NIH) [grant number EB016064].<ref name="Mueller2019" /> | ||
== Abstract and Conclusion == | == Abstract and Conclusion == | ||
'''Abstract'''<blockquote>Previous neuroimaging studies have detected markers of [[neuroinflammation]] in patients wit h [[ME/CFS|Myalgic Encephalomyelitis/Chronic Fatigue Syndrome]] (ME/CFS). | '''Abstract'''<blockquote>Previous neuroimaging studies have detected markers of [[neuroinflammation]] in patients wit h [[ME/CFS|Myalgic Encephalomyelitis/Chronic Fatigue Syndrome]] (ME/CFS). Magnetic Resonance Spectroscopy (MRS) is suitable for measuring [[brain]] metabolites linked to [[inflammation]], but has only been applied to discrete regions of interest in ME/CFS. We extended the MRS analysis of ME/CFS by capturing multi-voxel information across the entire brain. Additionally, we tested whether MRS-derived brain temperature is elevated in ME/CFS patients. Fifteen women with ME/CFS and 15 age- and gender-matched healthy controls completed fatigue and mood symptom questionnaires and whole-brain echo-planar spectroscopic imaging (EPSI). [[choline]] (CHO), myoinositol (MI), [[lactate]] (LAC), and N-acetylaspartate (NAA) were quantified in 47 regions, expressed as ratios over [[creatine]] (CR), an d compared between ME/CFS patients and controls using independent-samples t-tests. Brain temperature was similarly tested between groups. Significant between-group differences were detected in several regions, most notably elevated CHO/CR in the left anterior cingulate (p < 0.001). Metabolite ratios in seven regions were correlated with fatigue (p < 0.05). ME/CFS patients had increased temperature in the right insula, putamen, frontal cortex, thalamus, and the cerebellum (all p < 0.05), which was not attributable to increased [[body temperature]] or differences in cerebral perfusion. Brain temperature increases converged with elevated LAC/CR in the right insula, right thalamus, and cerebellum (all p < 0.05). We report metabolite and temperature abnormalities in ME/CFS patients in widely distributed regions. Our findings may indicate that ME/CFS involves neuroinflammation.<ref name="Mueller2019" /></blockquote>'''Conclusion'''<blockquote>This study is the first to investigate whole-brain MRS markers of [[neuroinflammation]] in ME/CFS. We report metabolite and temperature abnormalities in ME/CFS patients in widely distributed brain areas, suggesting ME/CFS is driven by diffuse pathophysiological processes affecting the whole [[brain]], rather than regionally limited, which is consistent with the heterogeneity of its clinical symptoms. Our findings add support to the hypothesis that ME/CFS is the result of chronic, low-level neuroinflammation. While the whole-brain results are preliminary, we note that they largely agree with past publications that use MRS in ME/CFS. These results should be replicated in future studies with larger samples to further establish the profile of pathophysiological abnormalities in the brains of ME/CFS patients. Ultimately, the development of sensitive MRI markers of ME/CFS could supplement clinical tests to help guide treatment decisions.<ref name="Mueller2019" /></blockquote> | ||
== Overview == | == Overview == | ||
The following information is provided by Solve ME/CFS Initiative.<ref name=" | The following information is provided by Solve ME/CFS Initiative.<ref name="RamsayAward20190117" /> | ||
'''''What you need to know:''''' | '''''What you need to know:''''' | ||
| Line 20: | Line 20: | ||
** Lactate (a byproduct of [[glycolysis]] in an oxygen-limited environment) was found to be increased in a number of brain areas, consistent with [[Neuroinflammation|brain inflammation]] and an [[energy deficit]] at the cellular level. | ** Lactate (a byproduct of [[glycolysis]] in an oxygen-limited environment) was found to be increased in a number of brain areas, consistent with [[Neuroinflammation|brain inflammation]] and an [[energy deficit]] at the cellular level. | ||
** Higher average temperatures were observed in five brain areas; the researchers included assessments that showed this finding wasn’t attributable to differences in blood flow or whole-body temperature. Inflammation requires more metabolic expenditures and three of the five areas also measured increased lactate, suggesting increased metabolism that could be related to neuroinflammation. | ** Higher average temperatures were observed in five brain areas; the researchers included assessments that showed this finding wasn’t attributable to differences in blood flow or whole-body temperature. Inflammation requires more metabolic expenditures and three of the five areas also measured increased lactate, suggesting increased metabolism that could be related to neuroinflammation. | ||
* The authors acknowledge a few limitations of the study, including the small sample size, but these preliminary results support a hypothesis of neuroinflammation in ME/CFS and provide a benchmark for replication using larger study groups.<ref name=" | * The authors acknowledge a few limitations of the study, including the small sample size, but these preliminary results support a hypothesis of neuroinflammation in ME/CFS and provide a benchmark for replication using larger study groups.<ref name="RamsayAward20190117" /> | ||
== Talks and interviews == | == Talks and interviews == | ||
* 2018, [https://www.youtube.com/watch?v=rxdzaWD5wfU ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study]<ref>{{Cite web|url=https://www.youtube.com/watch?v=rxdzaWD5wfU|title=ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study|date=Dec 14, 2018|access-date=|website=YouTube|last=|first=|authorlink=Jarred Younger|last2=|first2=|authorlink2=|archive-url=|archive-date=|dead-url=|publisher= | * 2018, [https://www.youtube.com/watch?v=rxdzaWD5wfU ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study]<ref name="Younger2018video">{{Cite web|url=https://www.youtube.com/watch?v=rxdzaWD5wfU|title=ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study|date=Dec 14, 2018|access-date=|website=YouTube|last=Younger|first=Jarred|authorlink=Jarred Younger|last2=|first2=|authorlink2=|archive-url=|archive-date=|dead-url=|publisher=[[Solve ME/CFS Initiative]]}}</ref> | ||
<gallery widths="200" heights="100" class="center" caption="Images from talk: "ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study""> | <gallery widths="200" heights="100" class="center" caption="Images from talk: "ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study""> | ||
| Line 32: | Line 32: | ||
== See also == | == See also == | ||
* [[Brain | * [[Brain#Inflammation|Brain inflammation]] | ||
* [[Jarred Younger]] | * [[Jarred Younger]] | ||
* [[Neuroinflammation]] | * [[Neuroinflammation]] | ||
* [[Solve ME/CFS Initiative]] | * [[Solve ME/CFS Initiative]] | ||
* [[Neurology of ME/CFS]] | |||
* [[List of abnormal findings in chronic fatigue syndrome and myalgic encephalomyelitis]] | |||
== Learn more == | == Learn more == | ||
* 2019, [http://go.solvecfs.org/webmail/192652/97033877/74d435097e87231f24cffe4d9de93da8e3f71c0e077a67417eba10cbd269ccb1 ''Brain Imaging and Behavior'' publication from Dr. Jarred Younger’s SMCI Ramsay pilot study supports involvement of neuroinflammation in ME/CFS]<ref name=" | * 2019, [http://go.solvecfs.org/webmail/192652/97033877/74d435097e87231f24cffe4d9de93da8e3f71c0e077a67417eba10cbd269ccb1 ''Brain Imaging and Behavior'' publication from Dr. Jarred Younger’s SMCI Ramsay pilot study supports involvement of neuroinflammation in ME/CFS]<ref name="RamsayAward20190117" /> | ||
== References == | == References == | ||
{{Reflist}} | |||
[[Category:Notable studies]] | [[Category:Notable studies]] | ||
Revision as of 18:51, February 28, 2021
Evidence of widespread metabolite abnormalities in Myalgicencephalomyelitis/chronic fatigue syndrome: assessmentwith whole-brain magnetic resonance spectroscopy is a 2019 research study of neuroinflammation in ME/CFS.
Authors[edit | edit source]
Christina Mueller (MS), Joanne Lin, Sulaiman Sheriff, Andrew Maudsley, under the direction of Dr. Jarred Younger of the Neuroinflammation, Pain and Fatigue Laboratory at University of Alabama at Birmingham and with University of Miami Miller School of Medicine radiology experts.[1][2]
Funding[edit | edit source]
This study was funded through Solve ME/CFS Initiatives' Ramsay Award Program[2][3] and the National Institutes of Health (NIH) [grant number EB016064].[1]
Abstract and Conclusion[edit | edit source]
Abstract
Previous neuroimaging studies have detected markers of neuroinflammation in patients wit h Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Magnetic Resonance Spectroscopy (MRS) is suitable for measuring brain metabolites linked to inflammation, but has only been applied to discrete regions of interest in ME/CFS. We extended the MRS analysis of ME/CFS by capturing multi-voxel information across the entire brain. Additionally, we tested whether MRS-derived brain temperature is elevated in ME/CFS patients. Fifteen women with ME/CFS and 15 age- and gender-matched healthy controls completed fatigue and mood symptom questionnaires and whole-brain echo-planar spectroscopic imaging (EPSI). choline (CHO), myoinositol (MI), lactate (LAC), and N-acetylaspartate (NAA) were quantified in 47 regions, expressed as ratios over creatine (CR), an d compared between ME/CFS patients and controls using independent-samples t-tests. Brain temperature was similarly tested between groups. Significant between-group differences were detected in several regions, most notably elevated CHO/CR in the left anterior cingulate (p < 0.001). Metabolite ratios in seven regions were correlated with fatigue (p < 0.05). ME/CFS patients had increased temperature in the right insula, putamen, frontal cortex, thalamus, and the cerebellum (all p < 0.05), which was not attributable to increased body temperature or differences in cerebral perfusion. Brain temperature increases converged with elevated LAC/CR in the right insula, right thalamus, and cerebellum (all p < 0.05). We report metabolite and temperature abnormalities in ME/CFS patients in widely distributed regions. Our findings may indicate that ME/CFS involves neuroinflammation.[1]
Conclusion
This study is the first to investigate whole-brain MRS markers of neuroinflammation in ME/CFS. We report metabolite and temperature abnormalities in ME/CFS patients in widely distributed brain areas, suggesting ME/CFS is driven by diffuse pathophysiological processes affecting the whole brain, rather than regionally limited, which is consistent with the heterogeneity of its clinical symptoms. Our findings add support to the hypothesis that ME/CFS is the result of chronic, low-level neuroinflammation. While the whole-brain results are preliminary, we note that they largely agree with past publications that use MRS in ME/CFS. These results should be replicated in future studies with larger samples to further establish the profile of pathophysiological abnormalities in the brains of ME/CFS patients. Ultimately, the development of sensitive MRI markers of ME/CFS could supplement clinical tests to help guide treatment decisions.[1]
Overview[edit | edit source]
The following information is provided by Solve ME/CFS Initiative.[2]
What you need to know:
- Dr. Jarred Younger and his co-authors completed a neuroimaging study of a female cohort of 15 individuals with ME/CFS (who met a modified Fukuda case definition) and 15 age-matched healthy controls using magnetic resonance spectroscopy (MRS).
- MRS, a type of MRI scan, provides a non-invasive method for evaluating the types and quantities of chemicals in the brain using 3D images and can give a readout of metabolic changes.
- The researchers found that “metabolite and temperature abnormalities were distributed across large portions of the brain” in ME/CFS participants, as compared to controls.
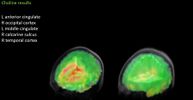
- The most significant finding was elevation of choline in the anterior cingulate (ACC) area of the brain on the left side. Increases in choline are associated with immune cell activation and the authors note that previous research indicates a critical role for the ACC region in cytokine-induced fatigue.
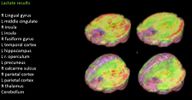
- Lactate (a byproduct of glycolysis in an oxygen-limited environment) was found to be increased in a number of brain areas, consistent with brain inflammation and an energy deficit at the cellular level.
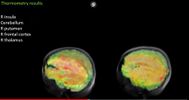
- Higher average temperatures were observed in five brain areas; the researchers included assessments that showed this finding wasn’t attributable to differences in blood flow or whole-body temperature. Inflammation requires more metabolic expenditures and three of the five areas also measured increased lactate, suggesting increased metabolism that could be related to neuroinflammation.
- The authors acknowledge a few limitations of the study, including the small sample size, but these preliminary results support a hypothesis of neuroinflammation in ME/CFS and provide a benchmark for replication using larger study groups.[2]
Talks and interviews[edit | edit source]
- Images from talk: "ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study"
Choline results: (L) ME/CFS patient (R) Healthy control patient. Image discussed @16:21
Lactate results: (L) ME/CFS patient (R) Healthy control patient. Image discussed @20:02
Thermometry results: (L) ME/CFS patient (R) Healthy control patient. Image discussed @26:00
See also[edit | edit source]
- Brain inflammation
- Jarred Younger
- Neuroinflammation
- Solve ME/CFS Initiative
- Neurology of ME/CFS
- List of abnormal findings in chronic fatigue syndrome and myalgic encephalomyelitis
Learn more[edit | edit source]
- 2019, Brain Imaging and Behavior publication from Dr. Jarred Younger’s SMCI Ramsay pilot study supports involvement of neuroinflammation in ME/CFS[2]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 Mueller, Christina; Lin, Joanne C; Sheriff, Sulaiman; Maudsley, Andrew A; Younger, Jarred W (2019). "Evidence of widespread metabolite abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy". Brain Imaging and Behavior. doi:10.1007/s11682-018-0029-4. Retrieved January 17, 2019. Cite has empty unknown parameter:
|dead-url=(help) - ↑ 2.0 2.1 2.2 2.3 2.4 "Brain Imaging and Behavior publication from Dr. Jarred Younger's SMCI Ramsay pilot study supports involvement of neuroinflammation in ME/CFS". go.solvecfs.org. Retrieved January 17, 2019. Cite has empty unknown parameter:
|dead-url=(help) - ↑ "Ramsay Award Program". Solve ME/CFS Initiative. Retrieved January 17, 2019.
- ↑ Younger, Jarred (December 14, 2018). "ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study". YouTube. Solve ME/CFS Initiative. Cite has empty unknown parameter:
|dead-url=(help)