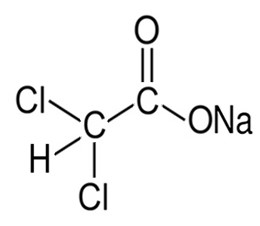
Dichloroacetate
Dichloroacetate or dichloroacetic acid or DCA is an analogue of acetic acid. Salts of DCA such as sodium dichloroacetate may be used as drugs because they inhibit pyruvate dehydrogenase kinase and boost or restore the function of mitochondria (cellular power plants).[1]
Sodium dichloroacetate[edit | edit source]
Sodium dichloroacetate is most popular and most researched form of DCA and was investigated in pilot trials as a possible treatment for ME/CFS. The first open-label pilot trial examined 22 patients (14 women, 8 men, mean age 43.3) suffering from long-lasting ME/CFS. The study found that 10 patients responded well, their score on the Fatigue severity scale decreased at least by 40%.[2]
Soon after the trial, members from of a ME/CFS community forum also shared their experiences with the substance, some of them positive.[3]
Molecular formulas[edit | edit source]
- C
2H
2Cl
2O
2
Uses[edit | edit source]
DCA, salts of dichloracetic acid, have been discovered in the 1960s. Since then the compound has been intensively researched. In 1983 it has been confirmed to be a potential remedy in treating congenital children mitochondrial disorders.[4]
Up till now, dozens of studies have proven that DCA can be useful in managing illnesses such as elevated serum cholesterol and triglycerides, diabetes, amyotrophic lateral sclerosis, pulmonary arterial hypertension, chronic fatigue syndrome, endometriosis and some forms of cancer.[2][5][6][7][8][9][10]
In 1989, Canada approved DCA for applying to the skin for treating warts and for "cauterization and removal of a wide variety of skin and tissue lesions", though this was cancelled post-market.[11]
Endometriosis[edit | edit source]
Recently, dichloroacetate has been described as a potential breakthrough treatment for endometriosis. It has been shown to reduce pain from endometriosis and also reduce the size of lesions associated with endometriosis.[12][13]
Currently sodium dichloroacetate is approved in Canada as an orphan-drug, the only way of treating children with inborn mitochondrial-errors. It is also used off-label in alternative cancer clinics in North America, South America and Europe.[14]
Theory[edit | edit source]
Sodium dichloroacetate is a molecule that can enter our cells. The living cells of most organisms have specialized structures that make chemical energy in our body, the mitochondria.
Once DCA enters the mitochondria, it inhibits the enzyme pyruvate dehydrogenase kinase. After this enzyme is halted, it stops blocking the pyruvate dehydrogenase enzyme. As a result this increases the energy output in our cells.[15]
It is thought that many sufferers of ME/CFS have mitochondrial dysfunction resulting from epigenetic, immunologic and inflammatory factors. Restoring the function of impaired mitochondria could potentially alleviate the symptoms of this disease.[2]
Evidence[edit | edit source]
Open-label pilot trial done in Belgium with 22 patients suffering from ME/CFS had favorable results. The Fatigue Severity Scale score decreased at least by 40% in 10 out of 22 patients.[2]
Anecdotal evidence from the members of Phoenix Rising forum, the results range from none, to positive or dramatic improvement.[3]
Formula used in the trials[edit | edit source]
DCA and its most popular form sodium dichloroacetate were used in the ME/CFS pilot trials. Both powder and capsules were found to be acceptable. The DCA purity used in the pilot trial for ME/CFS treatment was 99.9%.
The full nutraceutical formula was never revealed, however, based on the available literature it looked something like this:
- DCA 400 mg
- Vitamin B1 (thiamine) 100 mg
- Alpha-lipoic acid 100 mg
- Oxidoreductase ubiquinone (Q10) 50 mg
The formula was taken for 30 days straight. However, if symptom relief is not achieved after a month, DCA could be taken 5 days straight with a 2 day break at the end of the week. Researchers stated these cycles should be repeated every week for symptom control.
Risks and safety[edit | edit source]
Dichloroacetic acid is a strong acid and sodium dichloroacetate is the form closest to getting FDA-approved registration for other uses, and has the most extensively studied safety data.[16] Nevertheless, additional trials are needed to draw further conclusions.
A side effect reported by Comhaire (2018) was tremors.[2]
Costs and availability[edit | edit source]
DCA and its most popular form sodium dichloroacetate are available in naturopath clinics, as an off-label drug in Canada or on the internet. It is a non-patentable generic drug that can be freely transported all around the world.
Daily dose of DCA with the additional supplements in the nutraceutical formula cost about $1-2 daily.
Dichloroacetate is approved in Canada as an orphan-drug, and used off-label in alternative cancer clinics in North America, South America and Europe.[14]
Notable studies and publications[edit | edit source]
- 2018, Treating patients suffering from myalgic encephalopathy/chronic fatigue syndrome (ME/CFS) with sodium dichloroacetate: An open-label, proof-of-principle pilot trial[2] - (Full text)
- 2018, Why do some ME/CFS patients benefit from treatment with sodium dichloroacetate, but others do not?[17] - (Full text)
News and articles[edit | edit source]
- 2023, Researchers optimistic about potential new treatment for endometriosis - The Guardian
See also[edit | edit source]
Learn more[edit | edit source]
References[edit | edit source]
- ↑ "What is DCA ?". DCA Guide. Retrieved May 27, 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Comhaire, F (May 2018). "Treating Patients Suffering From Myalgic Encephalopathy/Chronic Fatigue Syndrome (ME/CFS) With Sodium Dichloroacetate: An Open-Label, Proof-Of-Principle Pilot Trial". Medical hypotheses. PMID 29602463. Retrieved May 21, 2020.
- ↑ 3.0 3.1 "DISCUSSION: Treating patients suffering from ME/CFS with sodium dichloroacetate (pilot trial)". Phoenix Rising ME/CFS Forums. Retrieved May 29, 2021.
- ↑ Stacpoole, Peter W.; Harman, Eloise M.; Curry, Stephen H.; Baumgartner, Thomas G.; Misbin, Robert I. (August 18, 1983). "Treatment of Lactic Acidosis with Dichloroacetate". New England Journal of Medicine. 309 (7): 390–396. doi:10.1056/NEJM198308183090702. ISSN 0028-4793.
- ↑ Moore, George W.; Swift, Larry L.; Rabinowitz, David; Crofford, Oscar B.; Oates, John A.; Stacpoole, Peter W. (July 1979). "Reduction of serum cholesterol in two patients with homozygous familial hypercholesterolemia by dichloroacetate". Atherosclerosis. 33 (3): 285–293. doi:10.1016/0021-9150(79)90180-1.
- ↑ Stacpoole, Peter W.; Moore, George W.; Kornhauser, David M. (March 9, 1978). "Metabolic Effects of Dichloroacetate in Patients with Diabetes Mellitus and Hyperlipoproteinemia". New England Journal of Medicine. 298 (10): 526–530. doi:10.1056/NEJM197803092981002. ISSN 0028-4793.
- ↑ Miquel, Ernesto; Cassina, Adriana; Martínez-Palma, Laura; Bolatto, Carmen; Trías, Emiliano; Gandelman, Mandi; Radi, Rafael; Barbeito, Luis; Cassina, Patricia (April 3, 2012). Ferreira, Sergio T. (ed.). "Modulation of Astrocytic Mitochondrial Function by Dichloroacetate Improves Survival and Motor Performance in Inherited Amyotrophic Lateral Sclerosis". PLoS ONE. 7 (4): e34776. doi:10.1371/journal.pone.0034776. ISSN 1932-6203. PMC 3318006. PMID 22509356.
- ↑ Michelakis, Evangelos D.; Gurtu, Vikram; Webster, Linda; Barnes, Gareth; Watson, Geoffrey; Howard, Luke; Cupitt, John; Paterson, Ian; Thompson, Richard B. (October 25, 2017). "Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients". Science Translational Medicine. 9 (413): eaao4583. doi:10.1126/scitranslmed.aao4583. ISSN 1946-6234.
- ↑ Leow, H. W.; Koscielniak, M.; Williams, L.; Saunders, P. T. K.; Daniels, J.; Doust, A.M.; Jones, M-C; Ferguson, G.D.; Bagger, Y. (December 2021). "Dichloroacetate as a possible treatment for endometriosis-associated pain: a single-arm open-label exploratory clinical trial (EPiC)". Pilot and Feasibility Studies. 7 (1): 67. doi:10.1186/s40814-021-00797-0. ISSN 2055-5784. PMC 7953373. PMID 33712086.
- ↑ Michelakis, E.D.; Sutendra, G.; Dromparis, P.; Webster, L.; Haromy, A.; Niven, E.; Maguire, C.; Gammer, T.L.; Mackey, J.R. (May 12, 2010). "Metabolic Modulation of Glioblastoma with Dichloroacetate". Science Translational Medicine. 2 (31): 31ra34–31ra34. doi:10.1126/scitranslmed.3000677. ISSN 1946-6234.
- ↑ "Dichloroacetic acid". DrugBank. Retrieved May 29, 2021.
- ↑ Horne, Andrew W.; Ahmad, S. Furquan; Carter, Roderick; Simitsidellis, Ioannis; Greaves, Erin; Hogg, Chloe; Morton, Nicholas M.; Saunders, Philippa T. K. (December 17, 2019). "Repurposing dichloroacetate for the treatment of women with endometriosis". Proceedings of the National Academy of Sciences. 116 (51): 25389–25391. doi:10.1073/pnas.1916144116. ISSN 0027-8424. PMC 6925989. PMID 31792175.
- ↑ Devlin, Hannah (March 8, 2023). "Researchers optimistic about potential new treatment for endometriosis". The Guardian. Retrieved March 9, 2023.
- ↑ 14.0 14.1 "Cancer Therapies". Arcadia Praxisklinik. Retrieved May 29, 2021.
- ↑ "How DCA works ?". DCA Guide. Retrieved May 29, 2021.
- ↑ "Phase 3 Trial of DCA in PDC Deficiency IND 028,625 (02/04/2015)". grantome.com. Retrieved May 29, 2012.
- ↑ Comhaire, Frank (November 1, 2018). "Why do some ME/CFS patients benefit from treatment with sodium dichloroacetate, but others do not?". Medical Hypotheses. 120: 65–67. doi:10.1016/j.mehy.2018.08.014. ISSN 0306-9877.