Neuroinflammation
Neuroinflammation is a term used to describe activation of the resident immune cells in the central nervous system (CNS).[1][2] This contrasts with classical Greco-Roman inflammation, which was originally defined as swelling, redness, heat, and pain, but has come to mean infiltration of tissues by blood-borne immune cells. Unlike classical inflammation, neuroinflammation does not imply infiltration of tissues by blood-borne immune cells. As such, the term “neuroinflammation” must not be confused with the term “encephalitis”, which implies classical inflammation. Because of this distinction, the relatively recent term “neuroinflammation” has generated considerable confusion in the scientific community.[3]
The immune cells activated in neuroinflammation are the tissue-resident macrophages of the CNS, which, for historical reasons, are called microglia.[1][4] Like other macrophages, microglia fight infections and repair tissue damage.[5] In the case of minor infections or minor tissue damage, microglia can often resolve the situation on their own. In more serious situations, the microglia will secrete cytokines to attract help from blood-borne immune cells.
Diseases associated with neuroinflammation[edit | edit source]
Neuroinflammation is a symptom of many diseases and thought to be a part of ME. Alzheimer's disease, Parkinson's disease, and multiple sclerosis are illnesses in which the brain experiences decline in structure and function, and also where it shows clear signs of neuroinflammation. Inflammation of the brain is linked to activated microglia, cytokine presence in the brain,[6] and changes in the neurochemicals produced by the brain.[7] These effects also occur in ME which is why researchers are searching to more strongly show neuroinflammation in these patients.
Causes[edit | edit source]
Microglia activation[edit | edit source]
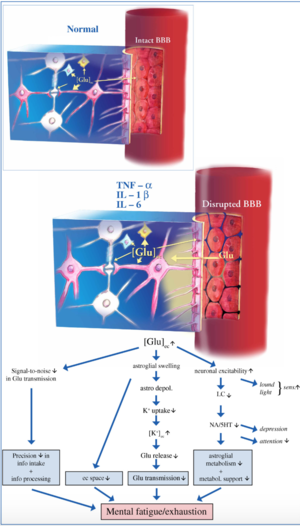
The blood brain barrier (BBB), a membrane that separates the brain from the rest of the body, may become compromised in ME patients. If there are cytokines circulating in the bloodstream, they may get into the brain through opened sections of the BBB[8]. While this initially starts as a normal brain response so that the brain can get the body back to normal, healthy functioning, this process can be predisposed to dysfunction and activation may be sustained longer than usual.
Microglia are cells that can act as the brain’s primary immune response. If cytokines or immune cells from outside the CNS enter the brain through the BBB, the microglia will respond to the immune threat and attempt to clear the infiltrators out. However, this process increases neuron activation and the release of more cytokines potentially leading to a cycle of neuroinflammation[8].
One study used a radioligand, a tracer that lights up in the presence of a specific molecule, in a positron emission tomography (PET) scanner in search of activated microglia in ME patients’ brains. Activated microglia cells are believed to be correlated to neuroinflammation. Increased radioligand presence in ME subjects’ brains was observed; however, further analysis of these data and replication of their results are needed[9].
Oxidative and nitrosative stress[edit | edit source]
The oxidative and nitrosative stress pathway or O&NS pathway results in tissue damage which could lead to neuroimflammation in ME/CFS.
Neuroinflammation may also be related to excess oxygen and nitrogen molecules in tissues. This can cause oxidative or nitrosative stress (O&NS), leading to tissue damage. The O&NS pathway helps maintain the blood brain barrier, an important membrane keeping the brain protected from harmful substances present in the blood. When the pathway is dysfunctional, the blood-brain barrier becomes less effective at keeping out particles. Breakdown of this barrier could lead to immune cells entering the brain and trigger an immune response, leading to neuroinflammation. Researchers propose a link between the dysfunction of brain tissues in ME/CFS and the breakdown of the oxidative and nitrosative stress pathway.[8]
Activation of cyclical neuroinflammation: A self-perpetuating cycle[edit | edit source]
When a patient gets an infection, the body attempts to return homeostasis. The immune system has regulatory structures called toll-like receptors (TLRs). High amounts of stress or a previous injury can predispose an individual’s TLRs to be more sensitive, releasing inflammatory molecules more readily in response to an immune stressor.[11] One of the downstream pathways of TLRs, the oxidative and nitrosative stress pathway (O&NS) can get activated. If this pathway is overstimulated, the body will produce a larger-scale response in an effort to return to normal.[12] In this attempt, a chemical called damage-associated molecular patterns (DAMPs) triggers the release of more inflammatory molecules, some of which activate the TLRs[13] (Morris et al., 2015). The process of activation from TLRs to the O&NS pathway to the production of more inflammatory molecules then becomes a cycle.[14]
Possible neurological biomarkers of ME[edit | edit source]
When the brain is going through challenges such as neuroinflammation or neurodegeneration, several chemicals become dysregulated. These changes are able to be recorded using a special function of magnetic resonance (MR) scanners. Because each chemical has a distinct molecular structure, the magnetic field formed by the scanner will bounce off of each chemical in unique ways. This allows the technician to measure the amounts of these chemicals in the brain.
Several neurochemicals have been studied in relation to ME patients. Myo-inositol is thought to be involved in astrocyte function (Albrecht et al. 2016) and trended to be higher in ME patients compared to controls.[15]
N-acetylacetate (NAA) shows neuron density, which has been found in other neurological disorders[7] and has been shown to be lower in ME patients,[14][15] but this was not found in all studies.[16][17]
Choline is linked to activation of glia, loss of energy and expression of macrophages in the brain[7] and has been shown to change compared to controls.[14][15][17] [18]
Lactate increases when more energy is being expended and has been shown to be higher than controls,[19][20][21][22] and significantly differs from lactate levels in people with psychological disorders.[19][22] Both ME patients and fibromyalgia patients were found to have similar levels of elevated lactate, so more tests would be needed to differentiate the two.[21]
Though contrasts were found between ME people and controls in many of these biomarker studies, researchers are not sure what the changes mean specifically because the metabolites are used in multiple brain processes. Furthermore, the results shown by these papers has not been largely replicated. However, if repeated, these biomarkers could potentially become an objective measure for diagnosing ME.
Notable studies[edit | edit source]
- 2010, Chronic fatigue syndrome: Harvey and Wessely's (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways[23] - (Full text)
- 2011, Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome[24] - (Abstract)
- 2010, Autopsies of four deceased ME patients showed various pathological phenomena in the CNS and peripheral nervous systems.[25]
- 2014, Brains of People With Chronic Fatigue Syndrome Offer Clues About Disorder. NY Times Well article by David Tuller on the brain scans of ME/CFS patient's researched by Stanford ME/CFS Initiative.[26][27][28]
- 2014, A Japanese PET study looked at neuroinflammation in 9 patients with ME/CFS and 10 controls. They measured a protein expressed by activated microglia, and found that values in the cingulate cortex, hippocampus, amygdala, thalamus, midbrain, and pons were 45%–199% higher in ME/CFS patients than in healthy controls. The values in the amygdala, thalamus, and midbrain positively correlated with cognitive impairment score, the values in the cingulate cortex and thalamus positively correlated with pain score, and the value in the hippocampus positively correlated with depression score.[29][30]
- 2016, Schizophrenia risk from complex variation of complement component 4[31]
- 2016, Reversal of cognitive decline in Alzheimer’s disease[32]
- 2017, Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study[33]
- 2019, Evidence of widespread metabolite abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy[34]
- 2019, Brain glial activation in fibromyalgia – A multi-site positron emission tomography investigation[35] - (Full text)
- 2020, In-vivo imaging of neuroinflammation in veterans with Gulf War illness[36] - (Full text)
Talks and interviews[edit | edit source]
- 2016, What is neuroinflammation?[37] - Jarred Younger
- 2016, Do you have a hot brain?[38] - Jarred Younger
- 2017, Brain on Fire with Dr. Mary Ackerley, MD
- 2017, Brain on Fire Webinar - Brain Changes in Mold Illness with Dr. Mary Ackerley
- 2018, ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study[39] - SolveCFS
See also[edit | edit source]
- Microglia
- List of abnormal findings in chronic fatigue syndrome and myalgic encephalomyelitis
- Jarred Younger
- Chronic pain
Learn more[edit | edit source]
- Mary Ackerley, MD
- Fibromyalgia, Chronic Fatigue Syndrome, Gulf War Illness – the Widespread Neuroinflammation Diseases - Cort Johnson
- 2018, Brain on Fire: Widespread Neuroinflammation Found in Chronic Fatigue Syndrome (ME/CFS)[40] - Cort Johnson
Younger’s new approach looked at the entire brain and found signs of inflammation almost everywhere. When asked what could cause that, Younger said that any neurodegenerative/ neuroinflammatory disorder like MS or a severe brain injury that tweaks the microglia (immune cells in the brain) enough to produce a sustained period of inflammation, burns up the oxygen in the system. Once that happens, the cells resort to anaerobic metabolism and lactate builds up just as it does in the muscles during exercise.
- 2014, Brain on Fire - The Role of Toxic Mold in Triggering Psychiatric Symptoms - Paradigm Change
References[edit | edit source]
- ↑ 1.0 1.1 Aguzzi, Adriano; Barres, Ben A.; Bennett, Mariko L. (January 11, 2013). "Microglia: Scapegoat, Saboteur, or Something Else?". Science (New York, N.Y.). 339 (6116): 156–161. doi:10.1126/science.1227901. ISSN 0036-8075. PMC 4431634. PMID 23307732.
- ↑ Mrak, Robert E.; Griffin, W. Sue T. (April 20, 2004). "Welcome to the Journal of Neuroinflammation!". Journal of Neuroinflammation. 1 (1): 1. doi:10.1186/1742-2094-1-1. ISSN 1742-2094. PMC 483051. PMID 15285806.
- ↑ Graeber, Manuel B.; Li, Wei; Rodriguez, Michael L. (December 1, 2011). "Role of microglia in CNS inflammation". FEBS letters. 585 (23): 3798–3805. doi:10.1016/j.febslet.2011.08.033. ISSN 1873-3468. PMID 21889505.
- ↑ Ginhoux, Florent; Greter, Melanie; Leboeuf, Marylene; Nandi, Sayan; See, Peter; Gokhan, Solen; Mehler, Mark F.; Conway, Simon J.; Ng, Lai Guan (November 5, 2010). "Fate mapping analysis reveals that adult microglia derive from primitive macrophages". Science (New York, N.Y.). 330 (6005): 841–845. doi:10.1126/science.1194637. ISSN 1095-9203. PMC 3719181. PMID 20966214.
- ↑ DiSabato, Damon J.; Quan, Ning; Godbout, Jonathan P. (October 2016). "Neuroinflammation: the devil is in the details". Journal of Neurochemistry. 139 (Suppl 2): 136–153. doi:10.1111/jnc.13607. ISSN 1471-4159. PMC 5025335. PMID 26990767.
- ↑ Chen, Wei-Wei; Zhang, Xia; Huang, Wen-Juan (April 2016). "Role of neuroinflammation in neurodegenerative diseases (Review)". Molecular Medicine Reports. 13 (4): 3391–3396. doi:10.3892/mmr.2016.4948. ISSN 1791-2997. PMC 4805095. PMID 26935478.
- ↑ 7.0 7.1 7.2 Albrecht, Daniel S.; Granziera, Cristina; Hooker, Jacob M.; Loggia, Marco L. (April 20, 2016). "In Vivo Imaging of Human Neuroinflammation". ACS chemical neuroscience. 7 (4): 470–483. doi:10.1021/acschemneuro.6b00056. ISSN 1948-7193. PMC 5433433. PMID 26985861.
- ↑ 8.0 8.1 8.2 Morris, Gerwyn; Maes, Michael (December 2013). "A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome". Metabolic Brain Disease. 28 (4): 523–540. doi:10.1007/s11011-012-9324-8. ISSN 1573-7365. PMID 22718491.
- ↑ Nakatomi, Yasuhito; Mizuno, Kei; Ishii, Akira; Wada, Yasuhiro; Tanaka, Masaaki; Tazawa, Shusaku; Onoe, Kayo; Fukuda, Sanae; Kawabe, Joji (June 2014). "Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An ¹¹C-(R)-PK11195 PET Study". Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine. 55 (6): 945–950. doi:10.2967/jnumed.113.131045. ISSN 1535-5667. PMID 24665088.
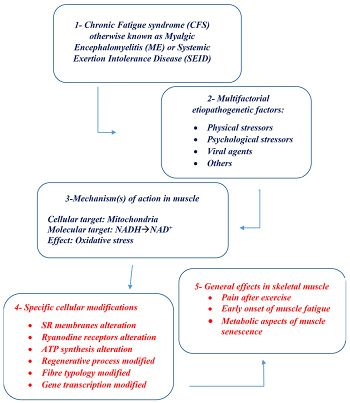
- ↑ Pietrangelo, Tiziana; Fulle, Stefania; Coscia, Francesco; Gigliotti, Paola Virginia; Fanò-Illic, Giorgio (September 7, 2018). "Old muscle in young body: an aphorism describing the Chronic Fatigue Syndrome". European Journal of Translational Myology. 28 (3). doi:10.4081/ejtm.2018.7688. ISSN 2037-7460.
- ↑ Gárate, Iciar; Garcia-Bueno, Borja; Madrigal, Jose Luis Muñoz; Caso, Javier Rubén; Alou, Luis; Gomez-Lus, Marisa L.; Micó, Juan Antonio; Leza, Juan Carlos (January 1, 2013). "Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway". Biological Psychiatry. 73 (1): 32–43. doi:10.1016/j.biopsych.2012.07.005. ISSN 1873-2402. PMID 22906518.
- ↑ Liu, JiaJun; Buisman-Pijlman, Femke; Hutchinson, Mark R. (2014). "Toll-like receptor 4: innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder". Frontiers in Neuroscience. 8: 309. doi:10.3389/fnins.2014.00309. ISSN 1662-4548. PMC 4179746. PMID 25324715.
- ↑ Morris, Gerwyn; Berk, Michael; Walder, Ken; Maes, Michael (February 6, 2015). "Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses". BMC Medicine. 13 (1). doi:10.1186/s12916-014-0259-2. ISSN 1741-7015. PMC 4320458. PMID 25856766.
- ↑ 14.0 14.1 14.2 Chaudhuri, A.; Condon, B.R.; Gow, J.W.; Brennan, D.; Hadley, D. M. (February 10, 2003). "Proton magnetic resonance spectroscopy of basal ganglia in chronic fatigue syndrome". Neuroreport. 14 (2): 225–228. doi:10.1097/01.wnr.0000054960.21656.64. ISSN 0959-4965. PMID 12598734.
- ↑ 15.0 15.1 15.2 Brooks, J.C.; Roberts, N.; Whitehouse, G.; Majeed, T. (November 2000). "Proton magnetic resonance spectroscopy and morphometry of the hippocampus in chronic fatigue syndrome". The British Journal of Radiology. 73 (875): 1206–1208. doi:10.1259/bjr.73.875.11144799. ISSN 0007-1285. PMID 11144799.
- ↑ Puri, B. K.; Counsell, S.J.; Zaman, R.; Main, J.; Collins, A.G.; Hajnal, J.V.; Davey, N.J. (November 2002). "Relative increase in choline in the occipital cortex in chronic fatigue syndrome". Acta Psychiatrica Scandinavica. 106 (3): 224–226. ISSN 0001-690X. PMID 12197861.
- ↑ 17.0 17.1 Tomoda, A.; Miike, T.; Yamada, E.; Honda, H.; Moroi, T.; Ogawa, M.; Ohtani, Y.; Morishita, S. (January 2000). "Chronic fatigue syndrome in childhood". Brain & Development. 22 (1): 60–64. ISSN 0387-7604. PMID 10761837.
- ↑ Puri, B.K.; Agour, M.; Gunatilake, K.D.R.; Fernando, K.A.C.; Gurusinghe, A.I.; Treasaden, I.H. (November 2009). "An in vivo proton neurospectroscopy study of cerebral oxidative stress in myalgic encephalomyelitis (chronic fatigue syndrome)". Prostaglandins, Leukotrienes, and Essential Fatty Acids. 81 (5–6): 303–305. doi:10.1016/j.plefa.2009.10.002. ISSN 1532-2823. PMID 19906518.
- ↑ 19.0 19.1 Mathew, Sanjay J.; Mao, Xiangling; Keegan, Kathryn A.; Levine, Susan M.; Smith, Eric L.P.; Heier, Linda A.; Otcheretko, Viktor; Coplan, Jeremy D.; Shungu, Dikoma C. (April 2009). "Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: an in vivo 3.0 T (1)H MRS imaging study". NMR in biomedicine. 22 (3): 251–258. doi:10.1002/nbm.1315. ISSN 0952-3480. PMID 18942064.
- ↑ Shungu, Dikoma C.; Weiduschat, Nora; Murrough, James W.; Mao, Xiangling; Pillemer, Sarah; Dyke, Jonathan P.; Medow, Marvin S.; Natelson, Benjamin H.; Stewart, Julian M. (September 2012). "Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology". NMR in biomedicine. 25 (9): 1073–1087. doi:10.1002/nbm.2772. ISSN 1099-1492. PMC 3896084. PMID 22281935.
- ↑ 21.0 21.1 Natelson, Benjamin H.; Vu, Diana; Coplan, Jeremy D.; Mao, Xiangling; Blate, Michelle; Kang, Guoxin; Soto, Eli; Kapusuz, Tolga; Shungu, Dikoma C. (2017). "Elevations of Ventricular Lactate Levels Occur in Both Chronic Fatigue Syndrome and Fibromyalgia". Fatigue: Biomedicine, Health & Behavior. 5 (1): 15–20. doi:10.1080/21641846.2017.1280114. ISSN 2164-1846. PMC 5754037. PMID 29308330.
- ↑ 22.0 22.1 Murrough, James W.; Mao, Xiangling; Collins, Katherine A.; Kelly, Chris; Andrade, Gizely; Nestadt, Paul; Levine, Susan M.; Mathew, Sanjay J.; Shungu, Dikoma C. (July 2010). "Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0 T. II: comparison with major depressive disorder". NMR in biomedicine. 23 (6): 643–650. doi:10.1002/nbm.1512. ISSN 1099-1492. PMID 20661876.
- ↑ Twisk, Frank; Maes, Michael (2010). "Chronic fatigue syndrome: Harvey and Wessely's (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways" (PDF). BMC Medicine. 8 (35).
- ↑ Maes, Michael; Twisk, Frank N.M.; Kubera, Marta; Ringel, Karl; Leunis, Jean-Claude; Geffard, Michel (February 2012). "Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome". Journal of Affective Disorders. 136 (3): 909–917. doi:10.1016/j.jad.2011.09.010.
- ↑ "Pathology of ME/CFS: pilot study of four autopsy reports". The ME Association. January 2011. Retrieved August 10, 2018.
- ↑ Tuller, David (November 24, 2014). "Brains of People With Chronic Fatigue Syndrome Offer Clues About Disorder". Well. Retrieved August 10, 2018.
- ↑ Zeineh, Michael M.; Kang, James; Atlas, Scott W.; Raman, Mira M.; Reiss, Allan L.; Norris, Jane L.; Valencia, Ian; Montoya, Jose G. (2015). "Right Arcuate Fasciculus Abnormality in Chronic Fatigue Syndrome". Radiology. 274 (2): 517–526. doi:10.1148/radiol.14141079. ISSN 0033-8419.
- ↑ "Study finds brain abnormalities in chronic fatigue patients". News Center. October 28, 2014. Retrieved September 22, 2018.
- ↑ Nakatomi, Yasuhito; Mizuno, Kei; Ishii, Akira; et al. (March 24, 2014), "Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An ¹¹C-(R)-PK11195 PET Study", Journal of Nuclear Medicine, 55 (6): 945-50, doi:10.2967/jnumed.113.131045, PMID 24665088
- ↑ Tuller, David (November 24, 2014), "Brains of People With Chronic Fatigue Syndrome Offer Clues About Disorder", NY Times
- ↑ Sekar, Aswin; Bialas, Allison R.; de Rivera, Heather; Davis, Avery; Hammond, Timothy R.; Kamitaki, Nolan; Tooley, Katherine; Presumey, Jessy; Baum, Matthew (February 11, 2016). "Schizophrenia risk from complex variation of complement component 4". Nature. 530 (7589): 177–183. doi:10.1038/nature16549. ISSN 1476-4687. PMC 4752392. PMID 26814963.
- ↑ Bredesen, Dale E.; Amos, Edwin C.; Canick, Jonathan; Ackerley, Mary; Raji, Cyrus; Fiala, Milan; Ahdidan, Jamila (June 2016). "Reversal of cognitive decline in Alzheimer's disease". Aging. 8 (6): 1250–1258. doi:10.18632/aging.100981. ISSN 1945-4589. PMC 4931830. PMID 27294343.
- ↑ Holmes, Sophie E.; Hinz, Rainer; Conen, Silke; Gregory, Catherine J.; Matthews, Julian C.; Anton-Rodriguez, Jose M.; Gerhard, Alexander; Talbot, Peter S. (January 1, 2018). "Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study". Biological Psychiatry. 83 (1): 61–69. doi:10.1016/j.biopsych.2017.08.005. ISSN 1873-2402. PMID 28939116.
- ↑ Mueller, Christina; Lin, Joanne C; Sheriff, Sulaiman; Maudsley, Andrew A; Younger, Jarred W (2019). "Evidence of widespread metabolite abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy". Brain Imaging and Behavior. 14 (2): 562–572. doi:10.1007/s11682-018-0029-4. Retrieved January 17, 2019.
- ↑ Albrecht, Daniel S.; Forsberg, Anton; Sandström, Angelica; Bergan, Courtney; Kadetoff, Diana; Protsenko, Ekaterina; Lampa, Jon; Lee, Yvonne C.; et al. (January 1, 2019). "Brain glial activation in fibromyalgia – A multi-site positron emission tomography investigation". Brain, Behavior, and Immunity. 75: 72–83. doi:10.1016/j.bbi.2018.09.018. ISSN 0889-1591.
- ↑ Alshelh, Zeynab; Albrecht, Daniel S.; Bergan, Courtney; Akeju, Oluwaseun; Clauw, Daniel J.; Conboy, Lisa; Edwards, Robert R.; Kim, Minhae; et al. (February 4, 2020). "In-vivo imaging of neuroinflammation in veterans with Gulf War illness". Brain, Behavior, and Immunity. 87: 498–507. doi:10.1016/j.bbi.2020.01.020. ISSN 0889-1591.
- ↑ Younger, Jarred (April 4, 2016). "What is neuroinflammation?". YouTube – via Younger Lab.
- ↑ Younger, Jarred (April 25, 2016). "Do you have a hot brain?". YouTube – via Younger Lab.
- ↑ "ME/CFS Involves Brain Inflammation: Results from a Ramsay Pilot Study". YouTube. SolveCFS. December 14, 2018.
- ↑ Johnson, Cort (September 24, 2018). "Brain on Fire: Widespread Neuroinflammation Found in Chronic Fatigue Syndrome (ME/CFS) - Health Rising". Health Rising. Retrieved September 26, 2018.