Ampligen
Rintatolimod (tradename Ampligen®) is a mismatched, double-stranded RNA molecule with immunomodulatory and antiviral properties. The drug acts as a TLR3 agonist which stimulates the production of interferons and tumor necrosis factors. It is manufactured by Hemispherx Biopharma.
It has been shown to raise natural killer cell function. [1]
History[edit | edit source]
Ampligen® was based on a double-stranded RNA (dsRNA) compound developed by the pharmaceutical company, Merck, in the 1960s, as a potential cancer drug. Though effective in the petri dish, the original compound proved to be too toxic for human use.[2][3]
William A. Carter, MD, a researcher at John Hopkins University, was able to modify the compound in the 1970s to reduce its toxicity (see section below on "Mechanism of action"). The new compound was named Ampligen®, short for “AMPLIfied GENetic activity.” In the 1980s, while a researcher at Hahnemann University in Philadelphia, Dr. Carter obtained the license for the compound from John Hopkins University. He and several other researchers at Hahnemann University affiliated with a small company, Hemispherx, now called Hemispherx Biopharma to manufacture it.[4]
In the late 1980s, Hemispherx Biopharma partnered with Dupont to start clinical trials for Ampligen®. After a couple years, Dupont severed its business relationship with Hemispherx Biopharma.[5]
Through the years, Dr. Carter's confidence in Ampligen®'s ability to stimulate the body's immune system lead to him offering the drug as a treatment for a variety of diseases including cancer, AIDS, chronic fatigue syndrome, hepatitis C, Gulf War Illness, swine flu, and ebola.[6][7] Hemispherx Biopharma stated: "...we believe that Ampligen® may have broad-spectrum anti-viral and anti-cancer properties."[8]
This over-confidence resulted in a case action suit by stockholders in 2013.[9]
Testing for efficacy in ME/CFS started in 1990 and continues to the present (see section below on "Drug approval status"). Testing for other conditions has been sporadic. In 2016, the University of Pittsburgh is sponsoring Phase I/II studies using Ampligen® as an adjunct treatment in ovarian, peritoneal, and colorectal cancer.[10] Hahnemann University and Hemispherx Biopharma are collaborating on Phase II studies for Ampligen® as a single agent for Renal Cell Carcinoma and Melanoma. Georgia Regents University is in the preclinical stage of testing Ampligen® as part of a combination therapy for Colorectal Cancer and Melanoma.[11]
In late fall 2015, Dr. Francis Collins announced that the NIH was considering the possibility of sponsoring a clinical trial for Ampligen®, as well as Rituximab and other treatments.[12] By the end of 2016, the NIH has not announced any further plans for a clinical trial for either drug.
At the 12th International IACFS/ME Research and Clinical Conference in October 2016, representatives from Hemispherx Biopharma announced "that a retrospective analysis of the AMP-516 Phase III trial of Ampligen® in patients with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME), segmented primarily by disease duration, showed that 51% of Ampligen® treated patients in a cohort with a disease duration of two to eight years vs. 18% of placebo patients demonstrated at least 25% improvement in placebo-adjusted exercise tolerance whereas the patient subset with less than two years or greater than eight years of disease duration failed to show a clinically-significant response."[13]
Marketing history[edit | edit source]
Mechanism of action[edit | edit source]
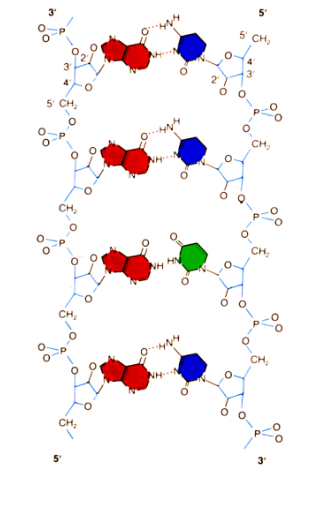
The chemical formation of Ampligen® begins with the known immunostimulant called Polyinosinic:polycytidylic acid (usually abbreviated Poly I:C). Poly I:C is a mismatched double-stranded RNA (dsRNA) with one strand being a polymer of inosinic acid and the other strand a polymer of cytidylic acid.[14]
Poly I:C is structurally similar to the type of dsRNA present in some viruses. When introduced into the body it stimulants the immune system because the body thinks a virus is present.
Ampligen® is formed when uridine (one of the five standard nucleosides which form the building blocks of RNA) is introduced into this Poly I:C strand, altering the strand to Poly I:C12U.[14]
As a result, one strand of the dsRNA is a homopolymer, poly rI and annealed to this strand is a heteropolymer, polyC12U.[14]
This alteration increases the compound's instability and shortens the half life to less than 40 minutes after IV administration. Plasma RNase (an enzyme naturally present in blood) degrades the Ampligen® compound into separate ribonucleotides which are completely natural degradation products the body can easily handle, thus lessening its toxicity.[14]
Ampligen® is a TLR3 agonist.[14] It stimulates the production of toll-like receptor 3 (TLR3), which is a naturally occurring protein. TLR3 recognizes the dsRNA present in some viruses, such as retroviruses, and stimulates a series of biochemical reactions that result is an increase in the production of interferon. Interferon, an important player in the body's immune system, protects against viral infections and activates immune cells, such as natural killer cells (NK cells).
Evidence[edit | edit source]
Coxsackie B[edit | edit source]
In a mouse model, Ampligen® was found to be protective of Coxsackie B3-induced myocarditis.[15]
HHV6[edit | edit source]
- 1994, A study done by the Department of Biochemistry, Temple University School of Medicine, Philadelphia, using IV therapy of Ampligen® "resulted in a significant decrease in HHV-6 activity (P < .01) and in downregulation of the 2-5A synthetase/RNase L pathway in temporal association with clinical and neuropsychological improvement."[16]
Efficacy in ME/CFS[edit | edit source]
- 2016, William M. Mitchell published a review in the journal, Expert Review of Clinical Pharmacology that stated: "Rintatolimod has achieved statistically significant improvements in primary endpoints in Phase II and Phase III double-blind, randomized, placebo-controlled clinical trials with a generally well tolerated safety profile and supported by open-label trials in the United States and Europe." Mitchell is the Chairman of the Board for Hemispherx Biopharma and a Professor of Pathology, Microbiology and Immunology at Vanderbilt University School of Medicine, Nashville, Tennessee.[17]
- 2015, Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME): Characteristics of Responders to Rintatolimod[18]
- 2015, David Strayer, et al., published "Low NK Cell Activity in Chronic Fatigue Syndrome (CFS) and Relationship to Symptom Severity," in the Journal of Clinical & Cellular Immunology. The study reviewed previous studies that concluded that the more decreased the Natural Killer cell cytotoxicity was in patients, the greater the CFS severity. The study, also, reported that in vitro exposure of peripheral blood mononuclear cells from CFS patients (fulfilling both the CDC 1988 and 1994 case definitions) to Ampligen® increased Natural Killer cell cytotoxicity 100-178%.[19]
- 2012, A double-blind, placebo-controlled, randomized, clinical trial of the TLR-3 agonist rintatolimod in severe cases of chronic fatigue syndrome[20]
- 2012, Hemispherx Biopharma presented several studies on efficacy at an FDA meeting:[14]
- The increase of baseline in mean exercise tolerance tests (ETT) improved 95.7% in the group on Ampligen® and 28.2% in the placebo group. (p value= 0.047; slide 81)[21]
- A greater percentage of Ampligen® patients (68.0%) decreased their use of concomitant medications used in an attempt to palliate symptoms of CFS compared to the placebo group (54.6%). (slide 59)[22]
- No Evidence for induction of autoantibodies with Ampligen® by assessment of Anti-dsDNA and Rheumatoid Factor in 64 randomly selected patients in controlled study AMP-516 at week 32; In the placebo group, 0% developed Anti-dsDNA and one patient (3.7%) developed Rheumatoid Factor autoantibodies. (slide 54)
- 2004, A study done at the Rega Institute for Medical Research, Belgium on coxsackie B3 virus-induced myocarditis in C3H/HeNHsd mice show Ampligen® markedly reduced the virus titers in the normalized heart electrocardiographic parameters[15]
- 2001, Chronic Fatigue Syndrome, Ampligen, and Quality of Life: A Phenomenological Perspective[23]
- 1995, Long Term Improvements in Patients with Chronic Fatigue Syndrome Treated with Ampligen®[24]
Drug approval status[edit | edit source]
United States[edit | edit source]
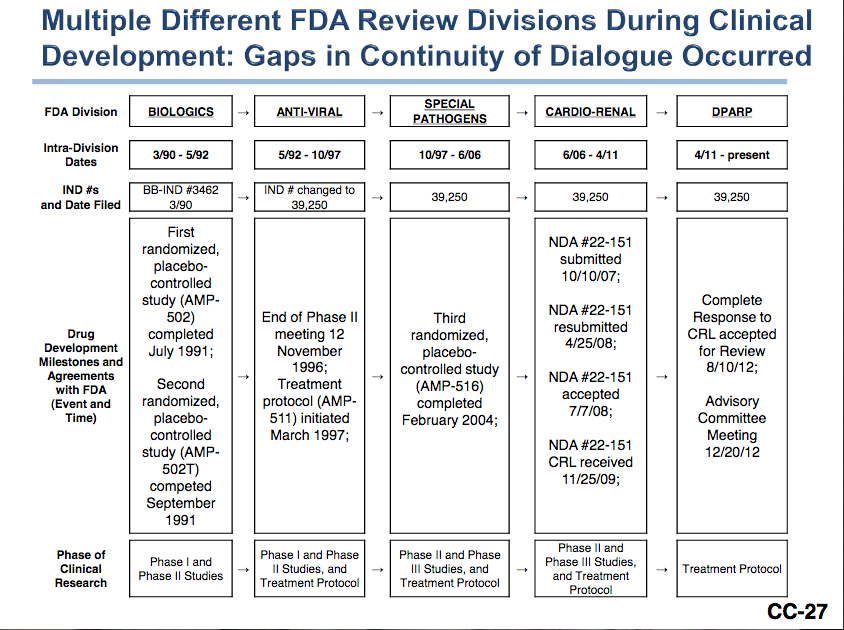
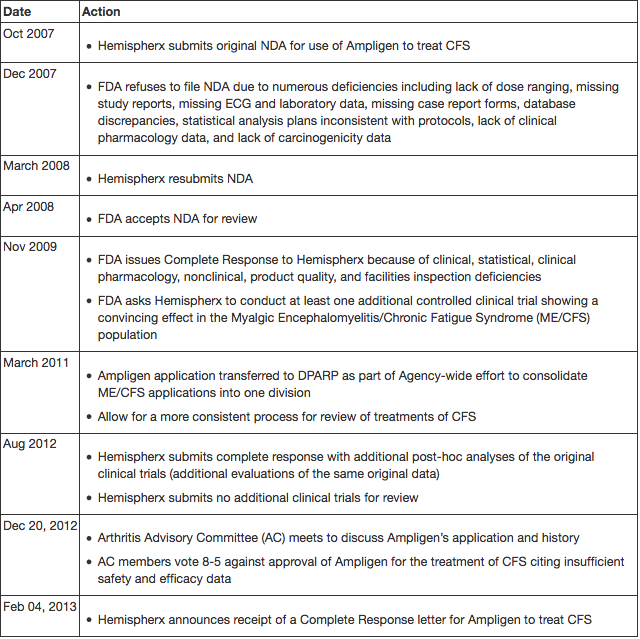
Ampligen® has been passed through five different FDA review divisions since 1990. Hemispherx Biopharma, Inc, wrote in a presentation to the FDA on Dec 20, 2012: "Most products have [the] same review Division during their entire development. Guidance from five (5) different Divisions [has provided] diverged/conflicting advice on major points, such as: interpreting primary endpoints (method of analysis), its collection and analysis, and different thresholds for determining toxicity and safety. In contrast, Hemispherx has had the same medical monitor for more than 20 years (Dr. David Strayer)."[14]
- History of Hemispherx Biopharma’s application to the FDA for Ampligen [25]
Europe[edit | edit source]
In 2016 Ampligen® started being made available on a limited basis in Europe.[26]
South America[edit | edit source]
Argentina (Argentine Republic) has approved the use of Ampligen® for ME/CFS on August 23, 2016. [27] Approval is limited to ME/CFS patients who are severely disabled and who have been diagnosed for more than one year.[11]
Notable studies[edit | edit source]
- 2016, Efficacy of rintatolimod in the treatment of chronic fatigue syndrome/ myalgic encephalomyelitis (cfs/me)
- 2015, Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop
- 2012, A double-blind, placebo-controlled, randomized, clinical trial of the TLR-3 agonist rintatolimod in severe cases of chronic fatigue syndrome.[20]
- 2004, The Interferon Inducer Ampligen [Poly(I)-Poly(C12U)] Markedly Protects Mice against Coxsackie B3 Virus-Induced Myocarditis[28] (Full Text)
- 1995, Long Term Improvements in Patients with Chronic Fatigue Syndrome Treated with Ampligen[24]
- 1994, Ampligen inhibits human herpesvirus-6 in vitro.[29]
- 1994, A controlled clinical trial with a specifically configured RNA drug, poly(I).poly(C12U), in chronic fatigue syndrome[30]
Experiences of patients on Ampligen®[edit | edit source]
- April, 1998 Testimony from Karen Lang
- October, 1998, Abstracts of Papers Presented at The Bi-Annual Research Conference of the American Association for Chronic Fatigue Syndrome (AACFS) - Four Patients of the Ampligen® 511 Cinical trial: Karen Lang, Linda Barossi, Steve Edwardsm and Stuart Craig Woolman
- 1999, Testimony before the Chronic Fatigue Syndrome Coordinating Committee (CFSCC) of the U.S. Department of Health and Human Services by patient, Mary M. Schweitzer, Ph.D.
- 2012, Jeannette Burmeister's comments to The Advisory Committee Reviewing Ampligen®
- 2012, Tell the FDA: What Have Your Experiences Been on Ampligen®?
- 2012-2013, The New Ampligen® Diaries by Kelvin Lord
- 2012, Anita Patton - What Ampligen® Means To Me
- 2013, Robert Miller's comments to HHS regarding being in a second clinical trial for Ampligen®
- 2013, Terry, an Ampligen® Patient
- 2016, My 22 years with Myalgic Encephalomyelitis (Mary Schweitzer)
Talks and interviews[edit | edit source]
Learn more[edit | edit source]
- Wikipedia - Rintatolimod
- 2016, Executive Informational Overview by Crystal Research Associates[11]
- 2016, Hemispherx hires Avrio as Ampligen® CMO while it tries to win US approval
- 2016, Making the Case for Ampligen® in Chronic Fatigue Syndrome (ME/CFS)
- 2016, Ampligen® and CFS
- 2016, Hemispherx Biopharma (HEB) Comments on Recent Meeting with NIH for ME/CFS Research Advancement (see also NIH Post-Infectious ME/CFS Study)
- 2016, Ampligen® Co-Inventor / Head of Hemispherx Biopharma Fired: Implications for ME/CFS Drug Unclear
- 2015, FDA Response Letter Regarding Approval of Ampligen® for ME/CFS
- 2015, Ampligen® price more than doubles - Available soon in Europe
- 2015, FDA Approval of MS Drug Puts Ampligen® Back In Play
- 2013, Ampligen® and biomarkers: my testimony to FDA Dec 2012
- 2013, Hemispherx Biopharma,Inc sued in a class action brought by stock holders claiming the company misrepresented whether Ampligen would be FDA approved. The stockholders prevailed.
- 2012, Ampligen® I: Effectiveness
- 2012, Slide presentation to FDA Arthritis Advisory Committee by Hemispherx [1]
- 2004, Mismatched double-stranded RNA: polyI:polyC12U
- 1994, The Aids Drug No One Can Have Mindy Kitei (see also Mindy Kitei)
- 1990, Chronic Fatigue Syndrome
See also[edit | edit source]
- Hemispherx Biopharma
- Nancy Kaiser patient 00
References[edit | edit source]
- ↑ http://www.omicsonline.org/open-access/low-nk-cell-activity-in-chronic-fatigue-syndrome-cfs-and-relationship-to-symptom-severity-2155-9899-1000348.php?aid=59415
- ↑ http://pomerantzlawfirm.com/assets/complaints/hemispherx.pdf
- ↑ http://phoenixrising.me/treating-cfs-chronic-fatigue-syndrome-me/immune/antivirals-and-immunemodulators/ampligen-rintatolimod/ampligen-i-effectiveness
- ↑ http://pomerantzlawfirm.com/assets/complaints/hemispherx.pdf
- ↑ http://phoenixrising.me/treating-cfs-chronic-fatigue-syndrome-me/immune/antivirals-and-immunemodulators/ampligen-rintatolimod/ampligen-part-ii-twisted-history
- ↑ http://www.bio-medicine.org/medicine-technology/Hemispherx-Presents-Evidence-of-Ampligen-Synergies-with-Existing-0AAntivirals-at-International-Avian-Influenza-Conference-453-1/
- ↑ http://www.wallstreetdaily.com/2014/11/06/hemispherx-biopharma-ebola-vaccine/
- ↑ http://www.hemispherx.net/
- ↑ http://pomerantzlawfirm.com/assets/complaints/hemispherx.pdf
- ↑ http://www.hemispherx.net/pipeline.php
- ↑ 11.0 11.1 11.2 Crystal Research Associates (November 27, 2016), Hemispherx Biopharma, Inc. (PDF) (Executive Informational Overview)
- ↑ http://www.meaction.net/2015/12/21/nih-considering-ampligen-and-rituximab-trials/
- ↑ https://globenewswire.com/news-release/2016/10/31/884673/0/en/Hemispherx-Biopharma-Announces-Identification-of-High-Responder-Patient-Subgroup-from-Ampligen-Phase-III-Trial-in-Patients-with-CFS-ME.html
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Hemispherx Biopharma, Inc. (December 20, 2012), Ampligen® for the Treatment of Chronic Fatigue Syndrome (PDF)
- ↑ 15.0 15.1 Padalko, Elizaveta; Nuyens, Dieter; De Palma, Armando; Verbeken, Erik; Aerts, Joeri L.; De Clercq, Erik; Carmeliet, Pete; Neyts1, Johan (2004), "The Interferon Inducer Ampligen [Poly(I)-Poly(C12U)] Markedly Protects Mice against Coxsackie B3 Virus-Induced Myocarditis", Antimicrobial Agents and Chemotherapy, 48 (1): 267–274, doi:10.1128/AAC.48.1.267-274.2004, PMID 14693549
- ↑ Suhadolnik, RJ; Reichenbach, NL; Hitzges, P; Sobol, RW; Peterson, DL; Henry, B; Ablashi, DV; Müller, WE; Schröder, HC; Carter, WA (January 1994), "Upregulation of the 2-5A synthetase/RNase L antiviral pathway associated with chronic fatigue syndrome.", Clinical Infectious Disease, 18 (Suppl 1): S96-104, PMID 8148461
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/27045557
- ↑ Strayer, David R; Stouch, Bruce C; Stevens, Staci R.; Bateman, Lucinda; Lapp, Charles W; Peterson, Daniel L; Carter, William A; Mitchell, William M (August 8, 2015), "Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME): Characteristics of Responders to Rintatolimod" (PDF), Journal of Drug Research and Development, 1 (1), doi:10.16966/2470-1009.103
- ↑ http://www.omicsonline.org/open-access/low-nk-cell-activity-in-chronic-fatigue-syndrome-cfs-and-relationship-to-symptom-severity-2155-9899-1000348.php?aid=59415
- ↑ 20.0 20.1 Strayer, DR; Carter, WA; Stouch, BC; Stevens, SR; Bateman, L; Cimoch, PJ; Lapp, CW; Peterson, DL; Chronic Fatigue Syndrome AMP-516 Study Group; Mitchell, WM (2012), "A double-blind, placebo-controlled, randomized, clinical trial of the TLR-3 agonist rintatolimod in severe cases of chronic fatigue syndrome.", PLoS One, 7 (3): e31334, doi:10.1371/journal.pone.0031334, PMID 22431963
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/22431963
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/22431963
- ↑ Christopher R. Snell, Staci R. Stevens & J. Mark Vanness. (2001). Chronic Fatigue Syndrome, Ampligen, and Quality of Life: A Phenomenological Perspective. Journal of Chronic Fatigue Syndrome, Vol. 8, Iss. 3-4, pp. 117-121. http://dx.doi.org/10.1300/J092v08n03_11
- ↑ 24.0 24.1 Strayer, DR; Carter, W; Strauss, KI; Brodsky, I; Suhadolnik, R; Ablashi, D; Henry, B; Mitchell, WM; Bastien, S; Peterson, D (1995), "Long Term Improvements in Patients with Chronic Fatigue Syndrome Treated with Ampligen", Journal of Chronic Fatigue Syndrome, 1 (1): 35-53
- ↑ http://www.fda.gov/Drugs/NewsEvents/ucm337759.htm
- ↑ Hemispherx ships Ampligen® for European chronic fatigue syndrome program
- ↑ Hemispherx Biopharma Announces Major Breakthrough: Approval for Commercial Sale of Rintatolimod (U.S. Tradename: Ampligen®) to Treat Severe Cases of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) in the Argentine Republic - NasDaq GlobeNewswire
- ↑ Elizaveta Padalko, Dieter Nuyens, Armando De Palma, Erik Verbeken, Joeri L. Aerts, Erik De Clercq, Peter Carmeliet, and Johan Neyts1. (2004). The Interferon Inducer Ampligen [Poly(I)-Poly(C12U)] Markedly Protects Mice against Coxsackie B3 Virus-Induced Myocarditis, Antimicrobial Agents and Chemotherapy, 48 (1): 267–274. doi:10.1128/AAC.48.1.267-274.2004
- ↑ Ablashi, DV; Berneman, ZN; Williams, M; Strayer, D; Kramarsky, B; Suhadolnik, RJ; Reichenbach, N; Hiltzges, P; Komaroff, AL (1994), "Ampligen inhibits human herpesvirus-6 in vitro", In Vivo, 8 (4): 587-91, PMID 7893986
- ↑ Strayer, DR; Carter, WA; Brodsky, I; Cheney, P; Peterson, D; Salvato, P; Thompson, C; Loveless, M; Shapiro, DE; Elsasser, W (1994), "A controlled clinical trial with a specifically configured RNA drug, poly(I).poly(C12U), in chronic fatigue syndrome", Clinical Infectious Diseases, 18 (Suppl 1): S88-95, PMID 8148460